A team of chemists from Oxford University and IBM Research Europe-Zurich has accomplished a significant milestone in the field of carbon allotropes. They have successfully synthesized a doubly anti-aromatic C16 carbon allotrope and published their findings in the esteemed journal Nature. This breakthrough has opened up new possibilities for exploring experimental theories in chemistry and the potential development of novel materials.
The Journey to C16 Carbon Allotrope
Before this discovery, a separate group of chemists had synthesized a different molecular form of carbon called C18. This allotrope was composed of a circular structure with alternating triple and single bonds between its atoms. The successful synthesis of C18 inspired the notion that it might also be possible to create a C16 carbon allotrope. However, previous attempts failed due to the inherent instability of the process.
Overcoming Challenges
In their efforts to synthesize the doubly anti-aromatic C16 carbon allotrope, the research team had to address the instability of the precursor molecules. These precursors possessed higher energies as a result of being anti-aromatic. To overcome this hurdle, the team employed several masking agents to improve stability during the synthesis process.
The final synthesis process involved utilizing a macrocycle containing two bromine and four carbon monoxide substituents. This macrocycle was deposited on a substrate of sodium chloride. To stimulate the formation of bonds between the carbon atoms, the researchers employed a tunneling microscope to send picoamperes of charge to specific sites on the molecule. This action increased the voltage on the bromine to 1.3V and the carbon monoxide to 3V, effectively forcing the desired bond formation.
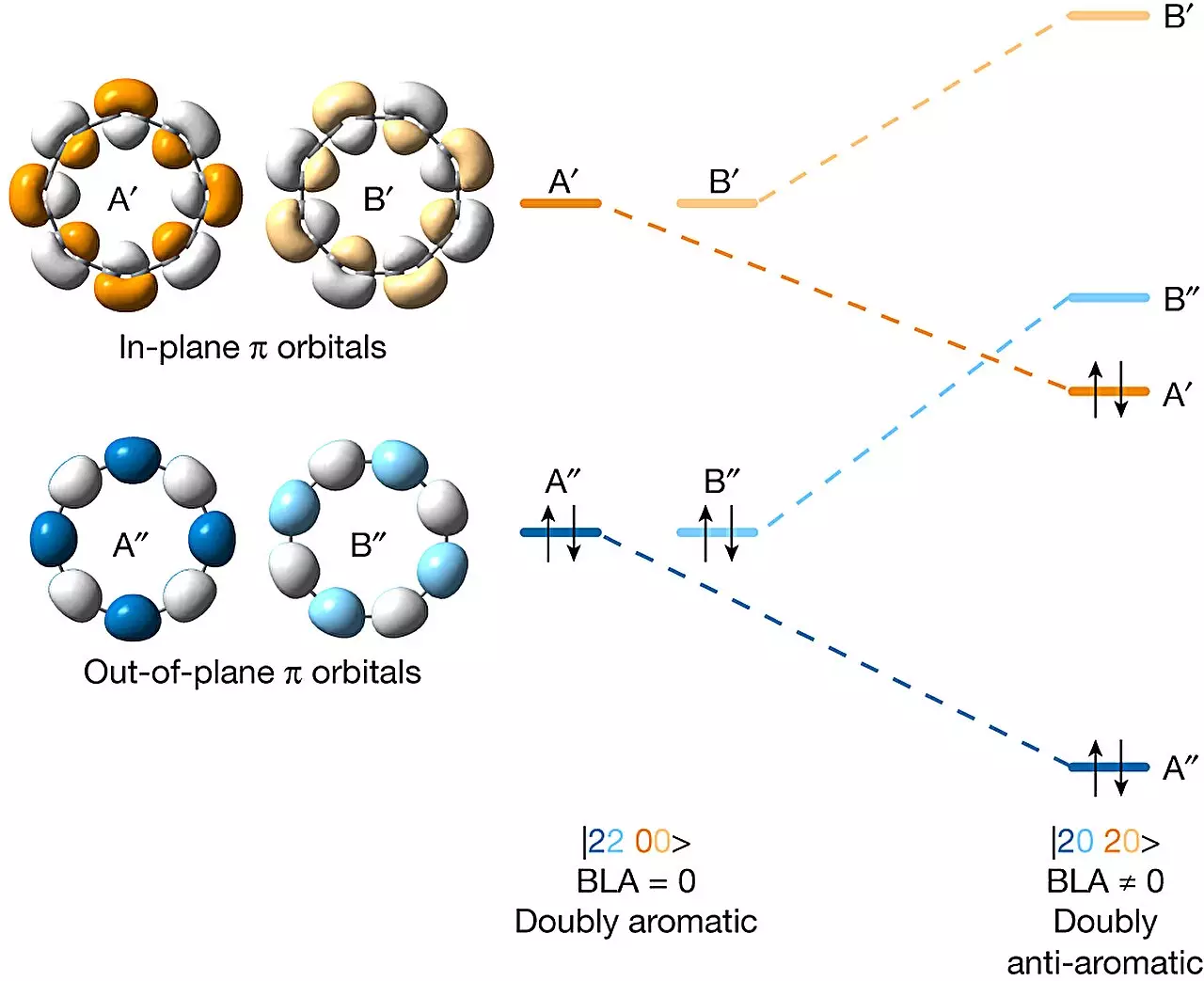
To confirm the successful creation of the desired allotrope, the researchers utilized an atomic force microscope to examine the created ring. Additionally, they employed a Kelvin probe force microscope and a scanning tunneling microscope to study the distribution of electrons in the molecular orbitals. This analysis verified that the molecule was indeed doubly anti-aromatic, aligning with their expectations.
Potential Implications
The synthesis of the doubly anti-aromatic C16 carbon allotrope has opened up new research opportunities in the field of chemistry. These exotic carbon allotropes may enable the exploration of new theories and concepts within the discipline. Furthermore, this breakthrough could potentially lead to the development of innovative and advanced materials with unique properties and applications.
The collaboration between chemists from Oxford University and IBM Research Europe-Zurich has resulted in the successful synthesis of a doubly anti-aromatic C16 carbon allotrope. Overcoming the challenges faced by previous attempts, the research team employed masking agents and precise manipulation techniques to achieve the desired outcome. This accomplishment holds great significance for the exploration of experimental theories in chemistry and the potential development of exotic carbon allotropes in the future.

Leave a Reply