Physical properties play a crucial role in the performance of pharmaceutical and functional materials. Their stability and solubility, among other factors, depend heavily on the solid-state form and environmental conditions. Recognizing the risks associated with physical instability, especially in life-saving medicines, the pharmaceutical industry has invested significantly in solid form screening platforms. However, quantitatively measuring free energy differences between crystalline forms has proven to be a challenge. Metastable crystal forms are difficult to prepare and often convert to more stable forms, making experimental data scarce. To overcome this limitation, experts in academia and industry have compiled the first-ever reliable experimental benchmark of solid-solid free energy differences for chemically diverse, industrially relevant systems. This groundbreaking work has paved the way for the use of computational methods in predicting these energy differences.
Determining free energy differences between crystalline forms is crucial for understanding and mitigating physical instability risks. However, the lack of reliable experimental benchmark data has hindered the development of accurate computational methods for prediction. Reports in the literature are sparse, and much of the experimental data on free energy determinations for pharmaceutical molecules remains unavailable to the public. This dearth of data poses a major bottleneck in advancing computational methods in the field.
A New Benchmark and Predictive Methods
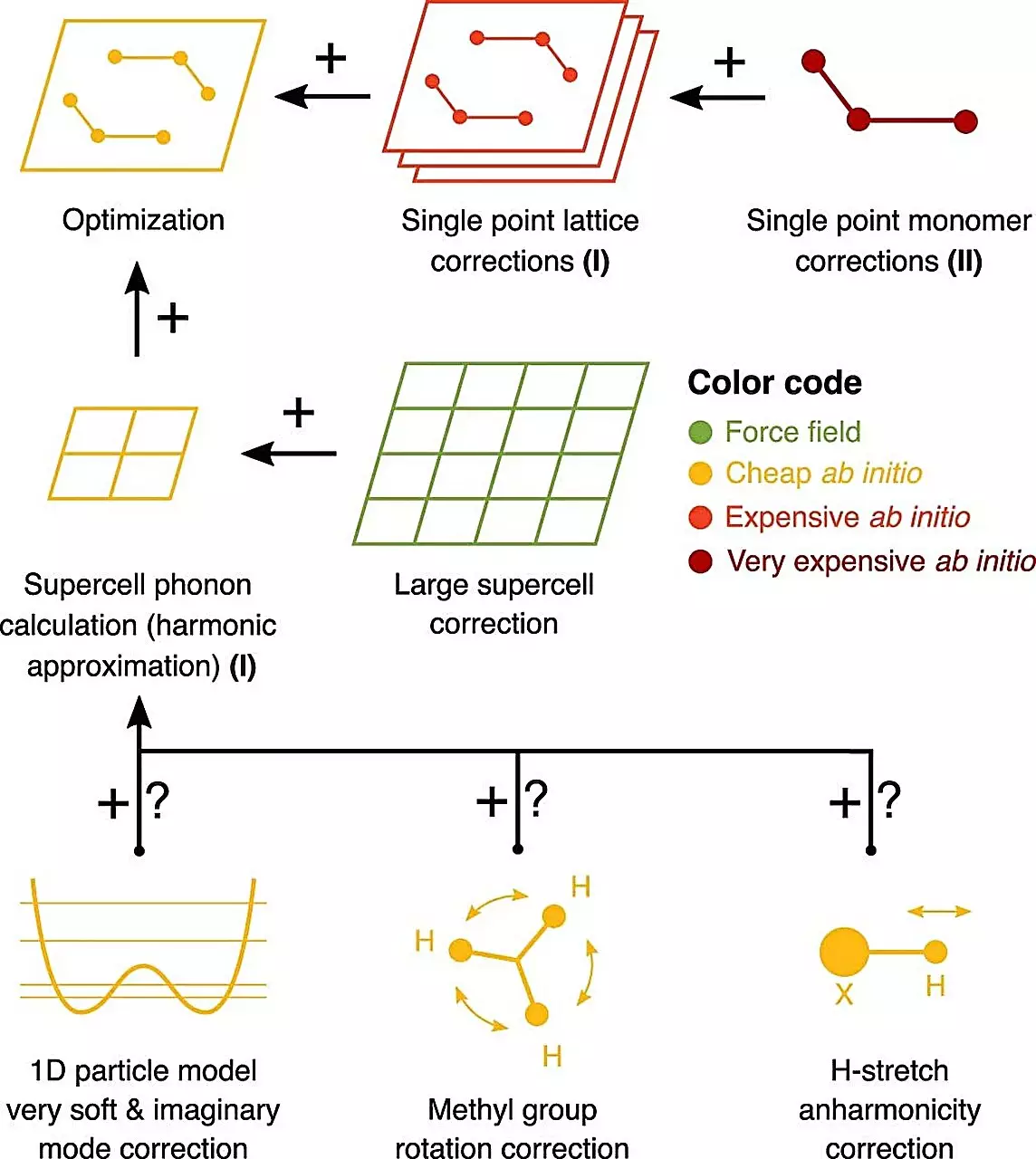
To address this challenge, a group of experts from academia and industry compiled a reliable experimental benchmark of solid-solid free energy differences for industrially relevant systems. This benchmark was then used to predict these energy differences using computational methods pioneered by Prof. Alexandre Tkatchenko’s group at the University of Luxembourg and further improved by Dr. Marcus Neumann and his team at Avant-garde Materials Simulation. What sets these predictive methods apart is their ability to leverage high-performance computing (HPC) without requiring any empirical input. Surprisingly, the calculations accurately predicted and explained data from seven pharmaceutical companies.
The potential implications of this work are vast, and it represents just one of the many potential applications of quantum mechanical calculations in the pharmaceutical industry. The ability to computationally model free energies opens doors for understanding and mitigating physical instability risks in all systems, even those that are experimentally challenging. This breakthrough has the potential to revolutionize the drug development process, minimizing the occurrence of disappearing polymorphs and market withdrawal of life-saving medicines. By accurately predicting solid-solid free energy differences, computational methods can aid in selecting stable forms and optimizing the performance of pharmaceutical and functional materials.
The Role of Computational Methods in Industry Innovation
The rapid adoption of computational methods in the pharmaceutical industry is a testament to the value they bring to research and industrial innovation. Prof. Tkatchenko, whose academic group developed the computational methods, expressed his thrill at witnessing their quick adoption. This collaboration between academia and industry has created an industrial working environment with an academic touch, fostering creativity based on core values such as honesty, integrity, perseverance, team spirit, and genuine care for people and the environment.
Bridging Science, Computing, and Industry for Health
The success of this endeavor illustrates the importance of building strong links between fundamental science, high-performance computing, and major industry players. By uniting these sectors, significant advancements can be made for the future of health. The computational methods employed in this research open up possibilities for further discoveries and innovations in the pharmaceutical industry. Prof. Jens Kreisel, Rector of the University of Luxembourg, emphasized the significance of this achievement and acknowledged the efforts in making a lasting impact for the future.
Computational methods have proven to be invaluable in predicting the energetics of drug crystal forms, overcoming the limitations of scarce experimental data. The ability to quantitatively measure free energy differences between crystalline forms enhances the understanding and mitigation of physical instability risks. This breakthrough has far-reaching implications for the pharmaceutical industry, enabling the selection of stable forms and optimizing the performance of pharmaceutical and functional materials. The collaboration between academia and industry, along with the utilization of high-performance computing, showcases the power of bridging science, computing, and industry for the betterment of healthcare.

Leave a Reply