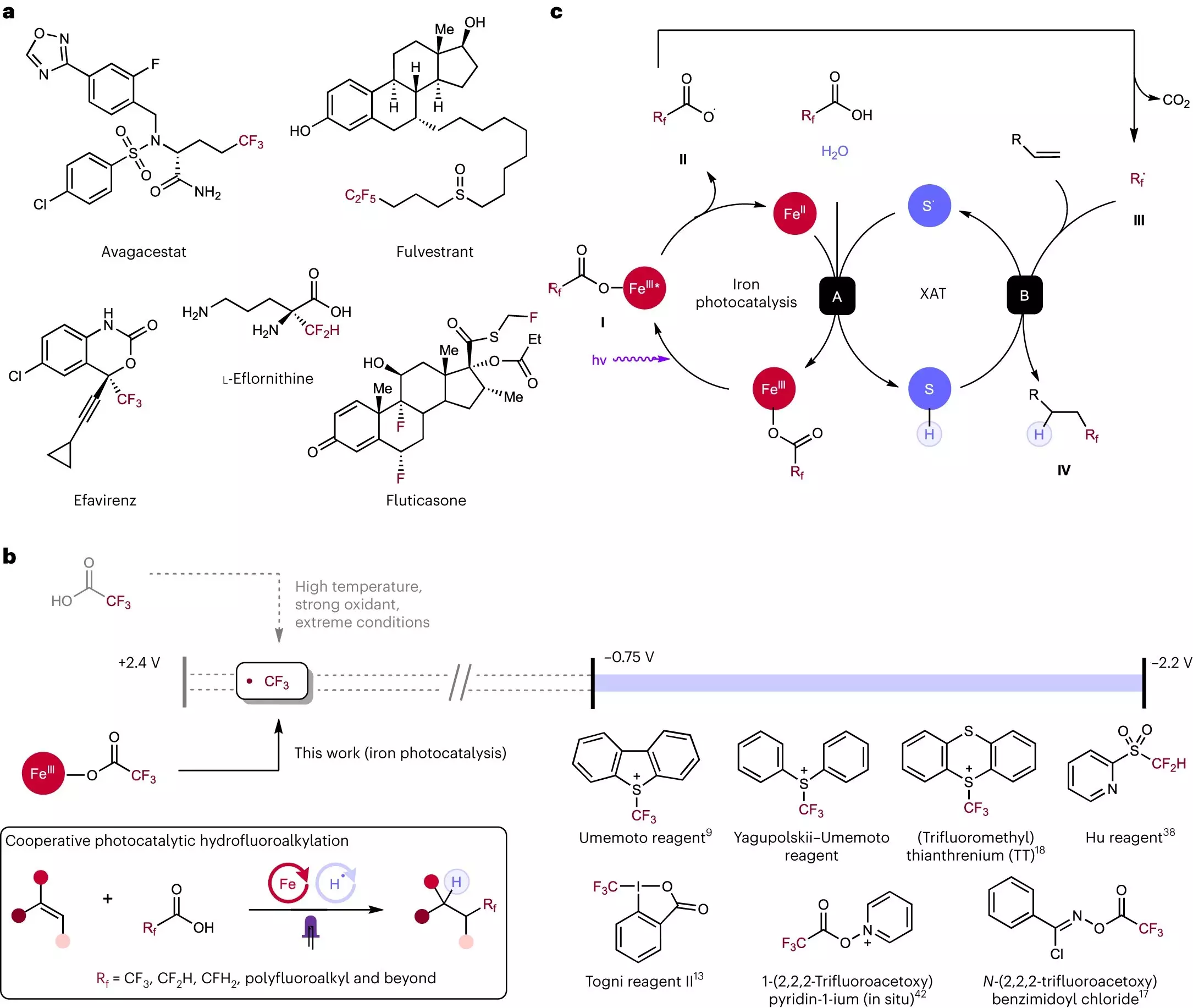
In the realm of chemical reactions, the element fluorine has gained a reputation as a “magic bullet atom” due to its outstanding ability to enhance drug absorption and extend the lifespan of pharmaceuticals. However, the conventional methods of incorporating fluorine into compounds have been hindered by their high cost and technical complexities. Addressing these limitations, scientists at Rice University have developed a cost-effective and reliable process for adding fluorine to molecules, thereby enhancing the efficiency of pharmaceutical drugs. This groundbreaking research, published in Nature Chemistry, demonstrates the potential of a light-activated iron and sulfur reaction to liberate fluorine from carboxylic acids and incorporate it into alkenes ⎯ common building blocks for pharmaceuticals and various chemical products. The novel chemical pathway formulated by the Rice researchers represents a revolutionary approach, offering easier, more affordable, and highly effective fluorination techniques.
Fluorine plays a pivotal role in the composition of many important drugs such as Prozac and Lipitor, significantly impacting their efficacy, duration, and potential side effects. By attaching fluorine atoms to drug molecules, the therapeutic effects can be accelerated, prolonged, and accompanied by fewer adverse reactions. Unfortunately, integrating fluorine into drug development processes has remained challenging. Existing methods often rely on expensive and highly reactive chemicals that yield unsatisfactory results. Recognizing the need for a cost-effective, efficient, and safe approach, the research team led by Julian West, the Norman Hackerman-Welch Young Investigator and assistant professor of chemistry, embarked on the pursuit of a groundbreaking solution.
Initially conceived as an ambitious idea, the research group set out to determine whether fluorine could be added to molecules using an economical, efficient, and safe process. Their efforts focused on exploring the possibility of utilizing elements readily available and abundant globally, such as iron and sulfur. In their earlier research conducted in 2019, the team discovered that when these elements were subjected to light, they interacted in a manner akin to unruly bar patrons, generating enough energy to dismantle carboxylic acid molecules. This process, known as decarboxylation, yielded fluorine atoms that could be utilized in other applications. Carboxylic acids, being prevalent, cost-effective, and resilient, proved to be ideal precursors for this transformative reaction.
Having successfully liberated fluorine atoms from stable compounds, the researchers faced the challenge of devising a process to reattach fluorine effectively and safely. Rice doctoral alum Yen-Chu Lu and chemistry graduate student Kang-Jie (Harry) Bian identified that fluorinated carboxylic acids could react with alkenes ⎯ another crucial component of drug molecules. Alkenes possess the versatility to be transformed into various entities, acting as a foundation for more complex drug structures. Through their investigations, Lu and Bian discovered that the same iron and sulfur combination utilized in cracking carboxylic acid molecules facilitated the attachment of the liberated fluorine fragments to alkenes.
One of the remarkable aspects of this newly developed method is its versatility, simplicity, and safety. The ability to incorporate different fluorine fragments is as straightforward as utilizing different carboxylic acids. To evaluate the robustness and boundaries of their technique, the researchers conducted extensive tests. Rice graduate student Shih-Chieh Kao, along with undergraduates Xiaowei Chen and David Nemoto Jr., collaborated with Lu and Bian to exert considerable stress on the strategy. These exhaustive investigations have demonstrated the remarkable potential of this approach, with yet unexplored frontiers waiting to be discovered.
The ground-breaking research conducted by the Rice University scientists offers a game-changing solution to the challenges associated with fluorine incorporation in pharmaceutical drug development. Their innovative use of light-activated iron and sulfur reactions marks a significant milestone in the field, introducing an easy-to-implement, cost-effective, and highly efficient method of fluorination. By overcoming the limitations of conventional techniques, this breakthrough has the potential to revolutionize the pharmaceutical industry and enhance the therapeutic value of drug molecules. The future holds great promise as further exploration and refinement of this approach may unlock even more possibilities for drug discovery and development.

Leave a Reply