Aspergillosis, a fungal infection caused by Aspergillus fumigatus, poses a significant threat to the lives of over 300,000 individuals each year. In a recent publication in Chemical Science, researchers from the University of Kansas have made significant strides in understanding the genes responsible for producing sartorypyrones, a chemical compound produced by A. fumigatus. This breakthrough could potentially lead to the development of more effective antifungal drugs, paving the way for improved treatment options for patients. In this article, we will delve into the research conducted by the investigators and explore the implications of their findings.
Fungal infections, including aspergillosis, have gained considerable attention due to their impact on public health. The majority of individuals affected by severe pathogenic fungal infections are immunocompromised, such as those undergoing cancer treatment or individuals living in sub-Saharan Africa without access to adequate medication. Aspergillus fumigatus, in particular, has emerged as a problematic organism, necessitating a deeper understanding of its secondary metabolites and their potential medicinal properties.
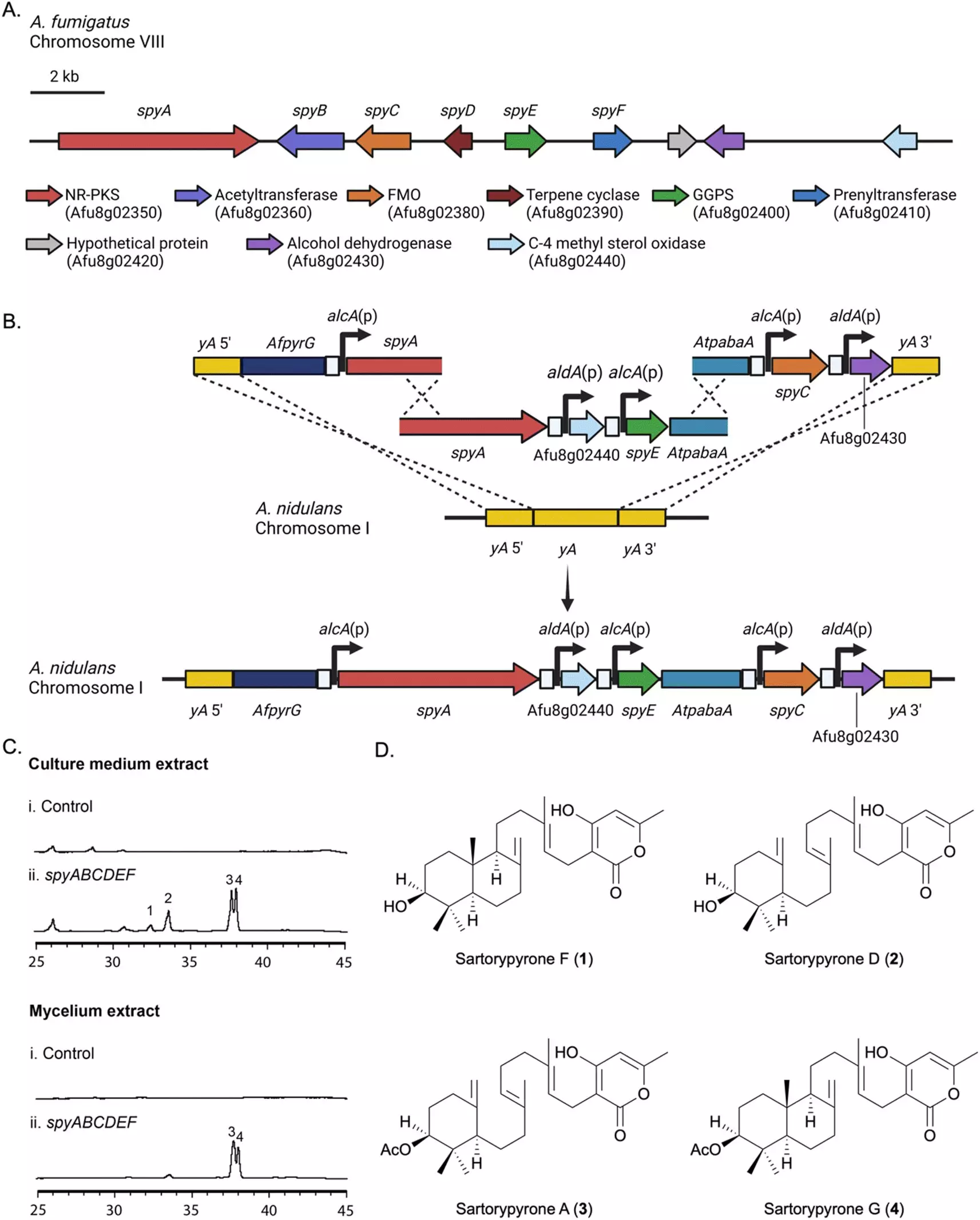
Secondary metabolites produced by fungi, such as A. fumigatus, have garnered interest for their bioactivity and potential therapeutic benefits. However, studying these compounds in the laboratory setting presents challenges as they are often not produced under standard conditions. The research team, led by Professor Berl Oakley, aimed to isolate and analyze the genes responsible for the production of secondary metabolites in A. fumigatus. To achieve this, they transferred a biosynthetic gene cluster (BGC) to a closely related strain of Aspergillus called A. nidulans.
Through their meticulous experimentation, the team successfully activated the transferred gene cluster, leading to the synthesis of sartorypyrones. This group of compounds, which had limited prior knowledge of their production, was analyzed using advanced techniques such as high-resolution electrospray ionization mass spectrometry, nuclear magnetic resonance, and microcrystal electron diffraction. The results revealed 12 chemical products, with seven of them being previously unidentified. The gene cluster responsible for these compounds was aptly named the “spy BGC” (sartorypyrones). The team also proposed a biosynthetic pathway for this family of compounds, providing valuable insights into their formation.
This breakthrough in deciphering the genes responsible for sartorypyrone production not only enhances our understanding of A. fumigatus but also presents potential therapeutic opportunities. The knowledge gained can pave the way for the development of new therapies for fungal infections and explore eco-friendly industrial applications. Professor Oakley’s previous research has showcased the potential of genetically modified A. nidulans in converting ocean plastics into raw materials for the pharmaceutical industry, highlighting the versatility of this approach.
While these findings are promising, Professor Oakley emphasizes the economic challenges associated with developing new antifungal medications. The development of profitable drugs poses constraints on the rapid translation of research into clinical applications. However, understanding the mechanisms of infection and the various secondary metabolite gene clusters could provide crucial insights for therapeutic interventions and limiting infection rates in the long run.
The groundbreaking research conducted at the University of Kansas shines a light on the genes responsible for producing sartorypyrones in Aspergillus fumigatus. This discovery holds great potential for the development of more effective antifungal drugs and a deeper understanding of fungal infections. By unraveling the biosynthetic pathway and identifying previously unknown compounds, researchers have laid a foundation for future investigations. While economic hurdles may impede the rapid development of new medications, the continued exploration of secondary metabolites and their role in pathogenesis will undoubtedly contribute to the advancement of treatment options for fungal infections.

Leave a Reply