Scientists at the Department of Energy’s Oak Ridge National Laboratory have recently made significant progress in the field of direct air capture (DAC). This innovative approach aims to remove carbon dioxide from the Earth’s atmosphere, with the ultimate goal of achieving negative emissions. The research, published in Cell Reports Physical Science, focuses on the foundational steps of carbon dioxide sequestration using aqueous glycine, a highly absorbent amino acid. By utilizing advanced computational methods, the scientists delved into the dynamic phenomena occurring in liquid solutions, particularly regarding the rate at which carbon dioxide can be captured.
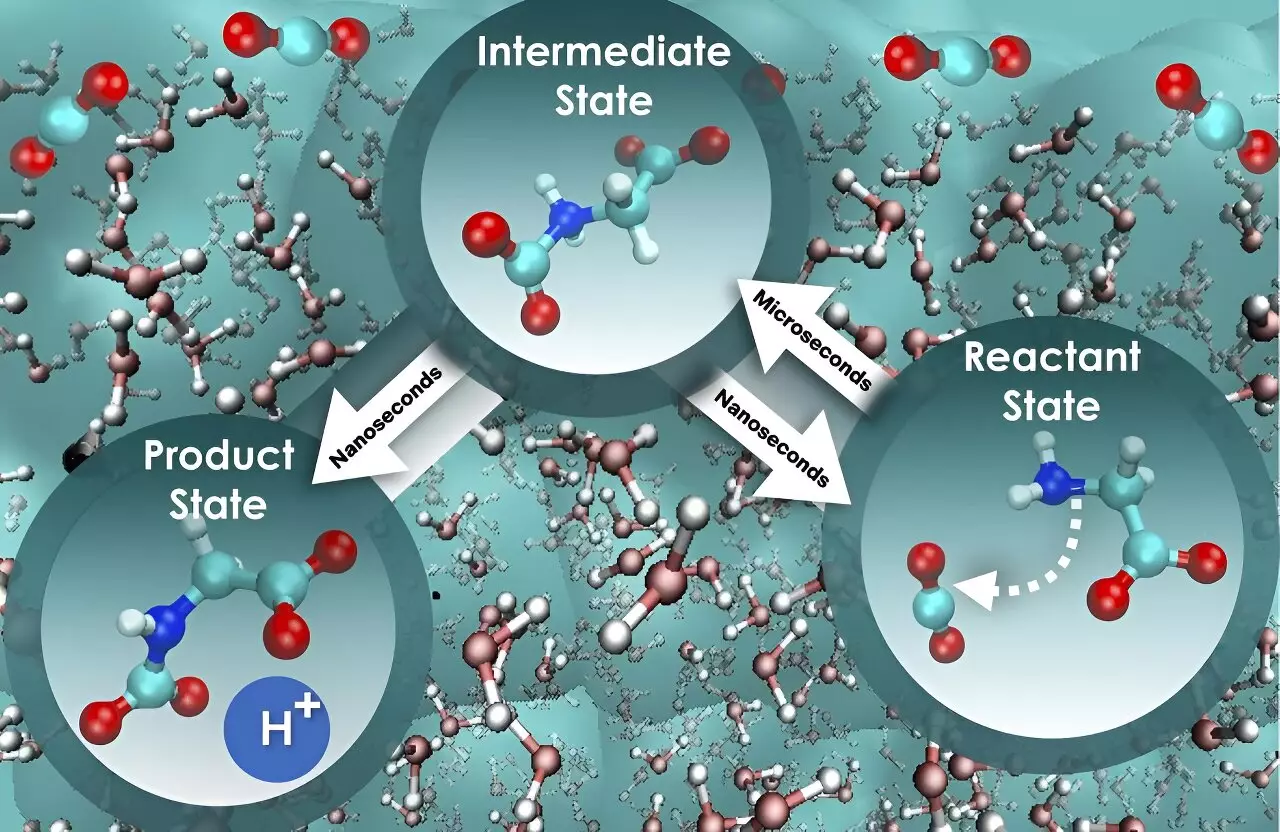
The process of chemical reactions in water is far from straightforward, especially when the motion of water molecules plays a significant role. Santanu Roy and Vyacheslav Bryantsev, the lead researchers, emphasized the need to understand the dynamic interactions, known as nonequilibrium solvent effects, to gain a comprehensive picture of reaction mechanisms and kinetics. By solely focusing on the free energy barrier as an indicator, the researchers discovered that the rate of carbon dioxide absorption cannot be accurately determined. This oversimplified approach fails to consider the role of water molecules and how they influence the speed of the reaction.
Through their extensive computational investigation, Roy and Bryantsev discovered intriguing insights into the carbon dioxide capture process. They found that the initial step, involving the interaction between glycine and carbon dioxide, occurs nearly 800 times slower than the subsequent step of releasing a proton to form a mixture of product state for holding the absorbed carbon dioxide. Although the free energy barrier remains constant for both steps, this unique perspective highlights the distinct speeds of these critical stages and provides a pathway to enhance the efficiency of carbon dioxide absorption and separation.
While the study’s findings are a significant advancement in understanding DAC, several challenges and future directions need to be considered. The ab initio molecular dynamics simulations used in this study were limited by their short time and length scales, as well as their high computational costs. To overcome these limitations, Xinyou Ma, one of the researchers, proposed combining a machine-learning approach with highly accurate simulations. This innovative approach would allow for molecular simulations at larger scales with reduced computational costs and provide a more accurate representation of chemical reactions.
Furthermore, examining the effects of macroscopic factors such as temperature, pressure, and viscosity on DAC is crucial for a comprehensive understanding of the process. By incorporating the attained molecular picture with these macroscopic factors, scientists can gain insights into the complex interplay between kinetics, thermodynamics, and molecular interactions in removing carbon dioxide from the atmosphere using aqueous amino acids.
Overall, this study sheds light on the intricate workings of DAC and emphasizes the vital role of kinetics and molecular interactions in carbon dioxide removal. As these mechanisms are better understood, the possibility of implementing large-scale DAC technology becomes more feasible. Currently, various DAC projects worldwide are at different stages of research, testing, and development. With continued advancements in the field, DAC holds great promise as a viable solution for combating climate change and reducing greenhouse gas emissions.

Leave a Reply