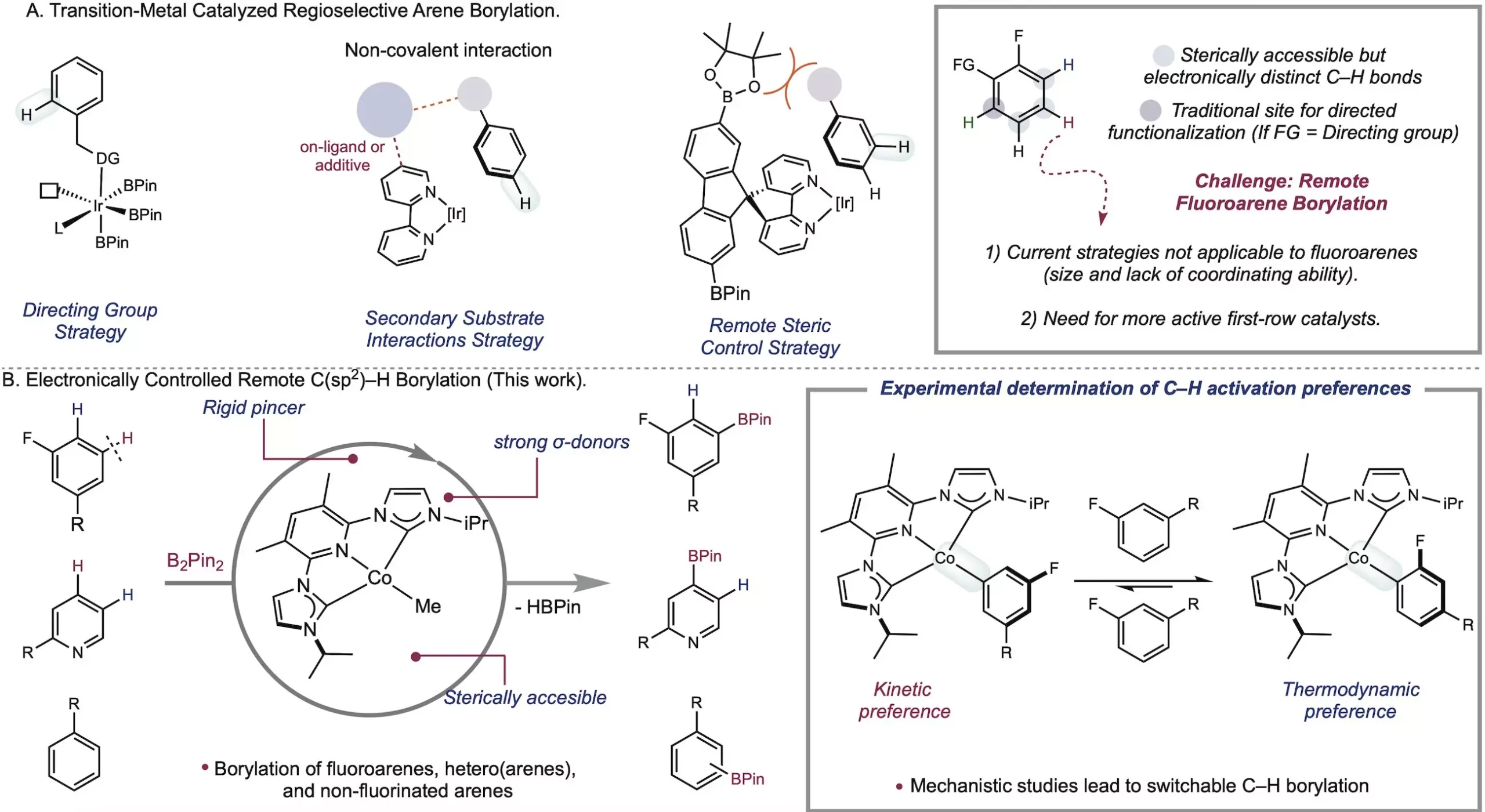
The field of metal-catalyzed C-H functionalization has long been plagued by the challenge of differentiating between bonds in fluoroarenes. However, the Chirik Group at the Princeton Department of Chemistry has taken a significant step forward by introducing a new method that leverages a cobalt catalyst to functionalize fluoroarenes based on their intrinsic electronic properties. Published in Science, their groundbreaking research eliminates the need for steric control and directing groups, allowing for meta-selective cobalt-catalyzed borylation. This innovative approach, driven by a deep understanding of organometallic chemistry, could revolutionize the synthesis of medicines, natural products, and materials. Additionally, the Chirik Lab’s method rivals the speed of iridium-based approaches, further cementing its potential as a game-changer in the field.
For decades, chemists have strived to make C-H bonds a reactive part of molecules, which holds immense promise for drug discovery and material synthesis. The Chirik Group’s focus on C-H borylation, the transformation of C-H bonds into carbon-boron bonds, opens up new avenues in synthetic chemistry. Among the various C-H bonds, the meta C-H bond has proven particularly challenging to selectively functionalize. However, through their work with a cobalt catalyst, the Chirik Group has achieved remarkable results that were previously unattainable.
Rational Design and Catalyst Development
At the core of the Chirik Group’s success lies their ability to rationally design catalysts and develop solutions based on fundamental organometallic principles. Through stoichiometric studies, they discovered that their catalyst exhibited high activity for C-H activation at room temperature. To harness this potential, the researchers designed a sterically accessible pincer ligand with rich electron density. This cleverly engineered ligand contributed to the catalyst’s enhanced reactivity and selectivity. By avoiding the use of aromatic solvents during the catalyst isolation process, the Chirik Lab effectively harnessed the power of their cobalt catalyst.
Expanding Beyond Iridium
While iridium has been extensively used as a catalyst for C-H functionalization, its limitations in selectively targeting specific C-H bonds have prompted the search for more sustainable alternatives. First-row transition metals like cobalt and iron offer cost-effective and environmentally friendly options. In 2014, the Chirik Lab pioneered the concept of electronically controlled C-H activation, enabling the differentiation between C-H bonds based on electronic properties. By tapping into the varying strengths of metal-carbon bonds, chemists can selectively functionalize desired bonds. Furthermore, the Chirik Group’s research uncovered a novel advantage: the possibility to switch site selectivity by exploiting the kinetic or thermodynamic preferences of C-H activation. This versatility allows for streamlined and cost-effective processes by choosing the appropriate reagent.
The achievement of site-selective meta-to-fluorine functionalization, a long-standing challenge in the field, stands as a testament to the Chirik Group’s groundbreaking research. By pushing the boundaries of first-row metals in C-H studies, they not only accomplished their initial goal but also discovered the ability to switch selectivity. This breakthrough revelation gives chemists the power to predict the outcome based on metal-carbon bond strengths, presenting a wealth of new opportunities. The Chirik Group’s cobalt catalyst represents a significant advancement in C-H functionalization and is poised to surpass the limitations of the iridium catalyst.
With the pivotal catalyst discovered by lead author Jose Roque, the Chirik Group is ready to delve further into the potential of borylation. Alex Shimozono, a postdoc in the lab, will play an instrumental role in driving new advances in this field. Their groundbreaking catalyst challenges the traditional notion that different site selectivities require distinct catalysts. By utilizing different boron sources, Roque demonstrated that meta-selective and ortho-selective borylation could be achieved using the same iPrACNCCo catalyst. This discovery further expands the toolbox available to chemists, enabling precise modifications in specific molecule sites.
The Chirik Group’s research marks a significant milestone in the field of metal-catalyzed C-H functionalization. By leveraging a cobalt catalyst and deep insights into organometallic chemistry, they have unlocked the potential of C-H borylation. This achievement not only eliminates the need for steric control and directing groups but also introduces a novel way to select specific C-H bonds based on their electronic properties. With their method rivaling the speed of iridium-based approaches and the ability to switch site selectivity, the Chirik Group’s work opens up a new era in C-H functionalization. The possibilities for synthesizing medicines, natural products, and materials have significantly expanded, setting the stage for groundbreaking developments in the future.

Leave a Reply