In a groundbreaking development, a research team at UNIST has successfully proven a previously theoretical method for producing a specific anticancer precursor substance. This discovery opens up new avenues for the development of innovative drugs and paves the way for extensive research on the effects of anticancer precursors on the human body.
Led by Professor Jaeheung Cho, the team from the Department of Chemistry at UNIST demonstrated that the synthesis of hydroxymato cobalt (III) can be achieved through the reaction of metal-active oxygen species with nitrile. Unlike previous studies that relied on expensive heavy metals, this novel method utilizes cost-effective metals and operates at lower temperatures, making it highly accessible and achievable.
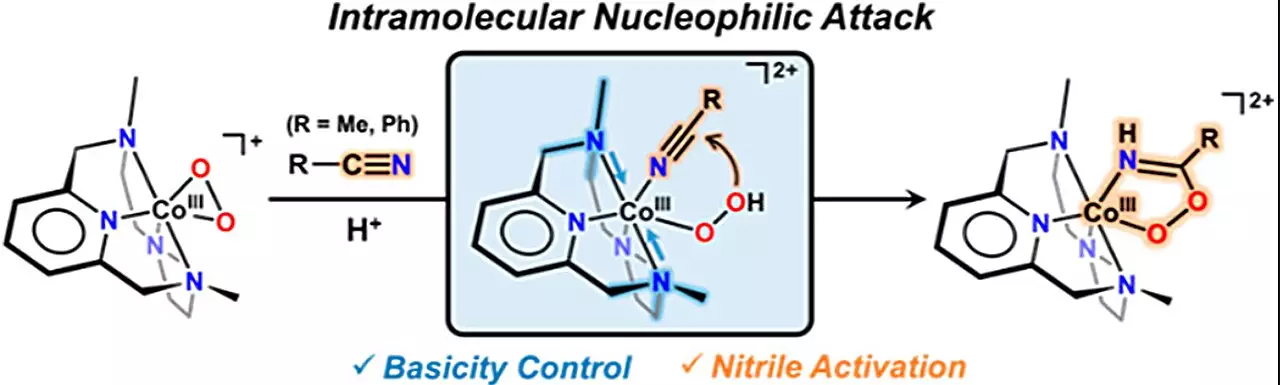
Nitrile, a compound widely used in pharmaceuticals and agricultural pesticides, has posed challenges in synthesis. However, the research team has discovered that the reaction between nitriles and cobalt-hydroperoxo species leads to the synthesis of peroxyimidateto cobalt (III). This finding of an intermediate substance formed during the chemical reaction is a significant step towards producing hydroxymiteto cobalt (III), the desired anticancer precursor.
Introduction of Acobalt(III)-Hydroperoxo Specifications
To synthesize cobalt (III)-peroxyimidato complexes, the research team introduced a new species called acobalt(III)-hydroperoxo specifications. The team observed that the reaction occurs through nucleophilic attack with nitrile. Additionally, the addition of a base to peroxymidato cobalt (III) transforms it into hydroxymito cobalt (III), facilitating the synthesis of precursors. This research places particular emphasis on the basicity of metal-dioxygen specifications, specifically the metal-(hydro)peroxo [M–O2(H)] complex species, which plays a crucial role in enabling rapid reactions even at low temperatures.
Insights from Computational Chemistry Simulations
To gain a better understanding of the structural aspects of cobalt(III)-hydroperoxo specifications, the research team employed computational chemistry simulations. These simulations allowed them to analyze the impact of changes in the combination of atoms on the structure of cobalt(III)-hydroperoxo specifications. The findings from these simulations reaffirm the vital role of basicity in the synthesis process.
This breakthrough in anticancer research marks a significant step forward in the development of innovative drugs. The novel method of producing selective anticancer precursor substances using cost-effective metals and lower temperatures provides new possibilities for advancing cancer treatments. The insights gained from this research will fuel further studies on the effects of anticancer precursors and contribute to the development of targeted therapies in the fight against cancer. With the potential for new drugs and treatments on the horizon, this research offers hope for a brighter future in the battle against cancer.

Leave a Reply