Ethylene, often referred to as the most important chemical in the petrochemical industry, is a fundamental building block for a wide range of everyday products. From antifreeze and vinyl to synthetic rubber, foam insulation, and various types of plastics, ethylene is utilized extensively. However, its current production method, known as steam cracking, is energy- and resource-intensive and releases significant amounts of carbon dioxide into the atmosphere. In recent years, researchers have been exploring an alternative known as oxidative coupling of methane (OCM), which has the potential to be a greener approach to ethylene production. Unfortunately, the yield of ethylene from OCM has not been economically viable. Nevertheless, a breakthrough catalytic development by researchers from North Carolina State University (NCSU) and Lehigh University is bringing OCM one step closer to industrial applicability.
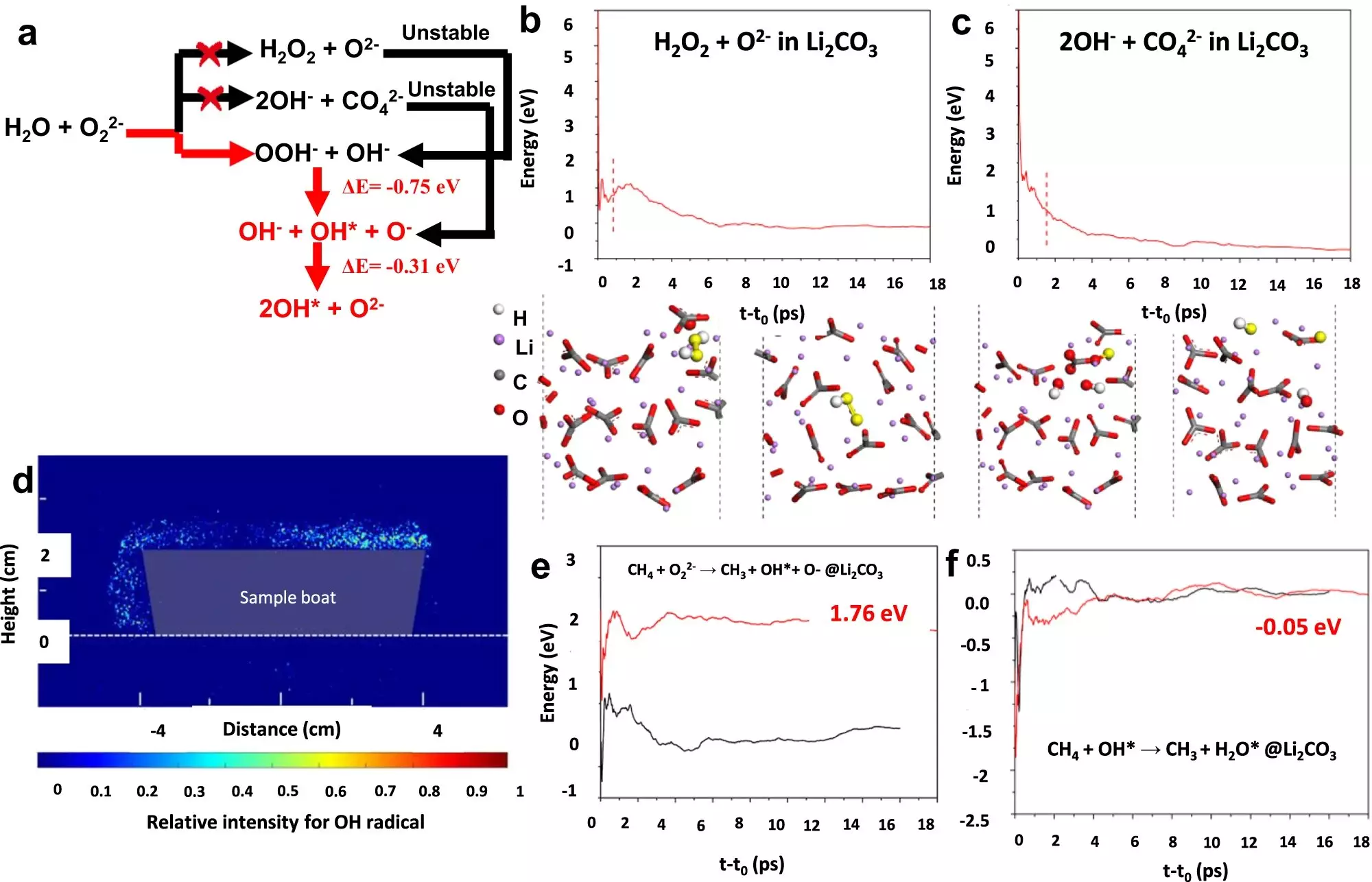
In a collaborative effort between NCSU, Lehigh University, the Guangzhou Institute of Energy Conversion, and the East China University of Science and Technology, a team of researchers successfully developed an OCM catalyst that demonstrates a yield of over 30% for ethylene production. The details of their breakthrough were published in Nature Communications. Led by Fanxing Li, Alcoa Professor of Engineering at NCSU, the team utilized a chemical looping scheme and developed a class of core-shell catalysts coated with Li2CO3 and mixed rare earth oxides. This innovative approach led to a single-pass yield of up to 30.6%, surpassing the economic viability threshold.
The key to achieving this high yield is the utilization of chemical looping, a sequential process of feeding methane and oxygen into the catalyst chamber. As explained by Bar Mosevitzky Lis, a postdoctoral research associate at Lehigh University, the catalyst undergoes a continuous cycle of oxidation and reoxidation. This allows for the replenishment of oxygen in the catalyst, ensuring its effectiveness throughout the reaction. By applying this chemical looping methodology, the researchers were able to optimize ethylene production.
The Lehigh University team, led by Israel Wachs, G. Whitney Snyder Professor of Chemical and Biomolecular Engineering, played a crucial role in the characterization of the catalyst. Specializing in in situ surface characterization, they employed a variety of physical and chemical techniques to study the catalyst’s surface transformations during the catalytic reaction. Their findings revealed that the high yield is a result of the interaction between the core and the shell of the catalyst during the chemical looping process.
The successful development of this catalyst brings the industry one step closer to affordable and sustainable ethylene production. Ethylene manufacturing through OCM has the potential to be more energy-efficient and emit fewer emissions compared to steam cracking. Furthermore, OCM utilizes methane, which can be sourced from natural gas, biogas, and potentially from the electrochemical reduction of carbon dioxide. The versatility of ethylene as a raw material opens up numerous possibilities for the creation of countless products, benefiting industries worldwide.
Although the breakthrough catalyst has shown promising results, the researchers acknowledge the need for further optimization and assessment of its suitability for large-scale production. The goal is to push the yield even higher, ensuring the economic viability and commercial appeal of OCM-mediated ethylene production. While challenges lie ahead, the fact that this breakthrough has surpassed earlier unfulfilled promises from the 1980s marks a significant milestone in the journey towards greener and more efficient ethylene manufacturing.
At the heart of this breakthrough lies the intricate dynamics and processes occurring within the catalyst. The core and shell of the catalyst undergo extreme transformations, generating fascinating surface phenomena. Through the collaborative efforts of multiple research institutions, the complexities of the catalyst system have been explored and harnessed, paving the way for a greener future in ethylene production.
The development of a high-yield catalyst for the oxidative coupling of methane brings us closer to a more sustainable and efficient method of producing ethylene. This groundbreaking breakthrough offers hope for a greener petrochemical industry and highlights the importance of continuous research and innovation in finding solutions to environmental challenges.

Leave a Reply