Minerals and mineralized tissue are not unusual in the natural world. However, the intricate formation of nacre, renowned for its mesmerizing iridescence and used in jewelry, has puzzled scientists for centuries. This composite material consists of biopolymers and platelets of crystalline calcium carbonate. Despite its beauty and significance, the exact processes that lead to nacre formation remain a subject of intense debate among experts. The formation of nacre begins with a mollusk extracting calcium and carbonate ions from its surroundings. Nevertheless, scientists have yet to uncover the molecular mechanisms behind its creation.
Unveiling the Role of ACC in Biomineralization
Amidst the uncertainty surrounding the formation of nacre, researchers have reached a significant breakthrough. In a recent study published in Nature Communications, a team of scientists from the University of Konstanz and Leibniz University Hannover has successfully unraveled the formation pathway of amorphous calcium carbonate (ACC). ACC is an essential non-crystalline intermediate involved in biomineralization processes. Lobsters and crustaceans, for instance, retain a supply of ACC in their stomachs, which they utilize to construct a new shell after molting.
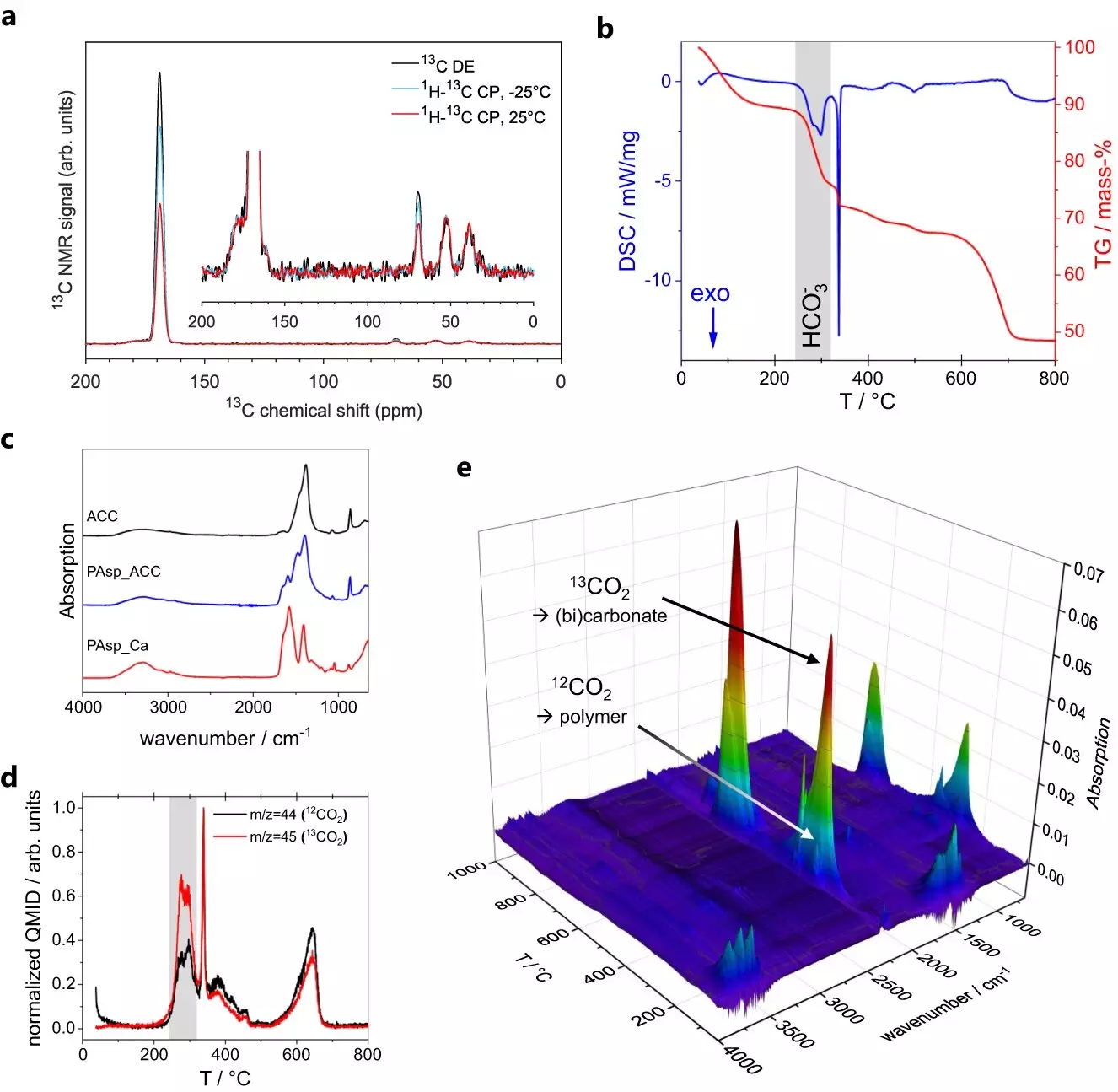
Utilizing advanced scientific techniques such as magic-angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy, the researchers scrutinized tiny ACC particles to discern their structural properties. The analysis of ACC posed a challenge due to its dynamic nature, which the scientists initially struggled to model effectively. However, a breakthrough emerged when Maxim Gindele from the Gebauer group at Leibniz University Hannover established that ACC exhibits electrical conductivity.
Measuring the electrical conductivity of ACC particles was not a straightforward task due to their fragility and minuscule size, measuring only tens of nanometers. The researchers employed a method called conductivity atomic force microscopy (C-AFM) to detect and visualize ACC particles on a flat surface. Through this technique, a minuscule cantilever scanned the surface, and a laser beam visualized the particles. By placing the cantilever on one of the nanoparticles, the scientists passed a current through its tip to measure the conductivity. Sanjay Vinod Kumar of the Mathies group at the University of Konstanz conducted further MAS NMR experiments to probe the dynamics of ACC. Their findings revealed the existence of two distinct chemical environments within the ACC particles.
The first chemical environment comprises calcium carbonate molecules with embedded water molecules that can only undergo 180-degree flips. Conversely, the second environment consists of water molecules that exhibit slow tumbling and translation, accompanied by dissolved hydroxide ions. The challenge faced by the researchers was reconciling these two chemical environments with the observed electrical conductivity. Traditionally, solid salts are insulators, and as such, the second, more mobile environment had to play a vital role in conducting electricity.
Formulating a New Structural Model
In light of their observations, the researchers postulated a new structural model for ACC. The mobile water molecules within ACC nanoparticles form a network, while the dissolved hydroxide ions carry the electrical charge. Moreover, the researchers shed light on the initial stages of biomineralization. In water, calcium and carbonate ions tend to form pre-nucleation clusters, dynamic assemblies that undergo phase separation. These dense, liquid droplets eventually merge into larger aggregations, resembling the merging of soap bubbles. The rigid, less mobile environment arises from the core of the dense, liquid nanodroplets, while the network of mobile water molecules emerges from imperfect coalescence during dehydration towards solid ACC.
The team’s findings represent a significant step forward in developing a structural model for ACC and shedding light on the overall process of biomineralization. The newfound understanding of pre-nucleation clusters and the formation of ACC opens doors to various practical applications. It may contribute to the development of cementitious materials capable of binding carbon dioxide. Furthermore, the discovery that ACC exhibits electrical conductivity opens exciting possibilities in the field of electrochemical devices.
The collaborative efforts of the scientists from the University of Konstanz and Leibniz University Hannover have propelled our knowledge of biomineralization forward. By unraveling the intricate formation pathway of ACC and shedding light on the enigmatic processes involved in the creation of nacre, this research promises new insights into the natural world and practical applications in various scientific disciplines.

Leave a Reply