The University of Münster has unveiled a groundbreaking strategy for transforming carbon–nitrogen atom pairs in a commonly used ring-shaped compound into carbon–carbon atom pairs. This groundbreaking method has the potential to revolutionize the search for active ingredients in new drugs. The research, led by Prof. Armido Studer from the Institute of Organic Chemistry, was published in Nature Chemistry.
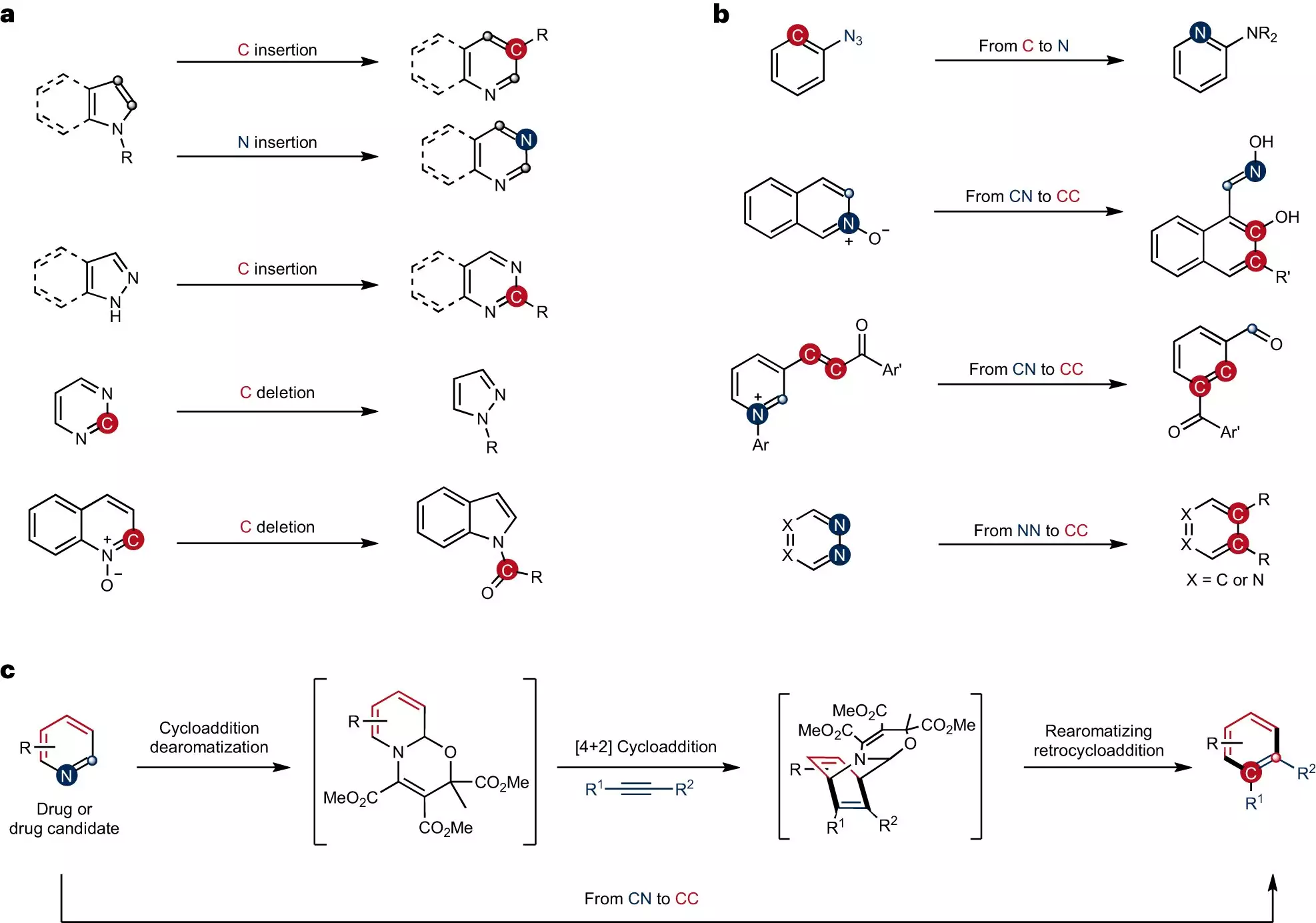
Chemists have long looked for ways to precisely alter ring-shaped structures. A method called skeletal editing has been used to accomplish this task by swapping individual atoms. However, until now, the conversion of carbon–nitrogen atom pairs in pyridines, a frequently used compound in synthesis, has remained elusive. This limitation has hindered progress in the development of new drugs and materials.
The innovative strategy devised by the research team involves converting carbon–nitrogen atom pairs in pyridines into carbon–carbon atom pairs. This approach opens up new possibilities for the synthesis of complex pyridines. To achieve this transformation, the team employed a two-step process. First, they carried out dearomatization, which changed the bonding structure of the pyridines, making them more reactive. The subsequent cycloaddition and rearomatization processes enabled the formation of skeletal-edited compounds.
Overcoming Inertness
One of the main challenges faced by the researchers was the inherent inertness of the pyridines used in the study. Modifying these compounds required altering their specific bonding structure through dearomatization. This process generated significantly more reactive intermediates, allowing for the subsequent formation of the desired compounds. Dr. Qiang Cheng, a post-doc on the team, explains, “We had to change their specific bonding structure through dearomatization in order to obtain significantly more reactive intermediates. The subsequent cycloaddition and rearomatization processes ultimately result in the formation of the skeletal-edited compounds.”
Synthetic Advancements
Through their new strategy, the research team successfully introduced synthetically valuable and medically significant functional groups to specific positions on the pyridine rings. This accomplishment was made possible by using a one-pot procedure, where the necessary reagents react with one another in a single vessel. This streamlined approach demonstrates the team’s ability to modify ring structures with precision, opening up new avenues for drug discovery and material development.
Theoretical Analysis
Dr. Christian Mück-Lichtenfeld from the Institute of Organic Chemistry conducted a thorough theoretical analysis of the reaction sequence’s mechanism. His insights provide a deeper understanding of the process and contribute to the overall advancement of the field. The combination of experimental findings and theoretical analysis strengthens the validity and significance of the team’s research.
The University of Münster’s breakthrough strategy for converting carbon–nitrogen atom pairs into carbon–carbon atom pairs in ring-shaped compounds holds tremendous potential for the discovery of new drugs and materials. By overcoming the challenges of skeletal editing, the research team has paved the way for more precise modifications of complex pyridines. The introduction of synthetically valuable functional groups to specific positions on ring structures is a significant achievement that will shape the future of organic synthesis. This innovative approach represents a major leap forward in the field of chemistry and offers exciting possibilities for scientific advancements.

Leave a Reply