Recent groundbreaking research has shed light on an exciting new avenue for potential therapeutic intervention in breast and gynecological cancers. Findings published in the prestigious Journal of Medicinal Chemistry have highlighted the promising target MYT1 and unveiled a series of potent and highly selective inhibitors designed specifically to tackle this protein. Supported by Insilico Medicine’s pioneering AI-driven generative biology and chemistry engine, this study demonstrates the immense potential of computational approaches in advancing cancer research.
Breast and gynecological cancers continue to exert a severe toll on the lives of countless women worldwide, adversely impacting their health, fertility, and overall quality of life. Recognizing the urgent need for novel therapeutics, the dedicated research team harnessed the power of Insilico’s proprietary AI-driven target identification platform, PandaOmics. Through meticulous analysis of comprehensive data encompassing five distinct types of gynecological cancers, including ovarian, endometrial, cervical, and breast cancer, the study identified a common denominator that transcended these diseases: MYT1.
MYT1, a member of the Wee1-kinase family, exhibits minimal expression in normal cells but becomes highly expressed in most types of cancer. Intriguingly, this study found MYT1 to be consistently significant across all analyzed diseases, highlighting its relevance as a potential therapeutic target. Cell cycle regulation and the vital role of MYT1 inhibition, particularly concerning the synthetic lethality of CCNE1 amplification, emerged as a key focus, suggesting MYT1’s potential as a promising strategy in combating cancers characterized by genome instability.
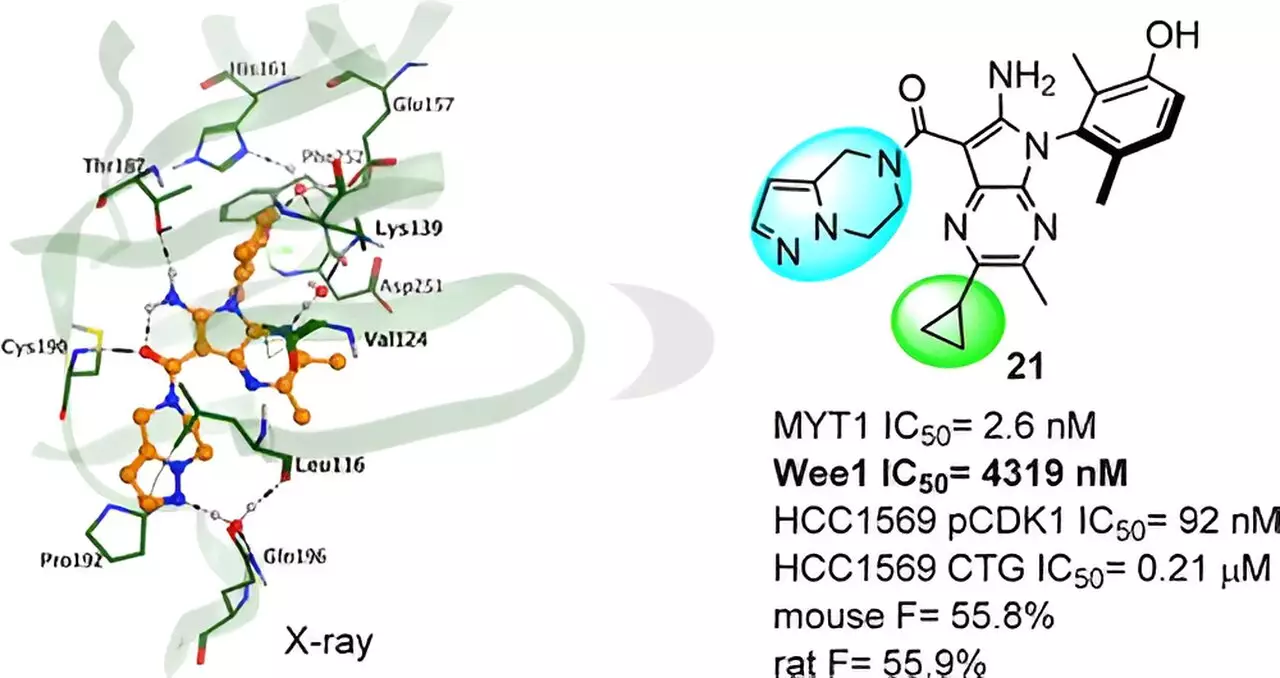
One of the primary challenges in developing selective MYT1 inhibitors is its striking homology to Wee1. This similarity poses a significant obstacle for researchers striving to design compounds that exclusively target MYT1. However, the study addressed this pressing issue through the utilization of Insilico’s AI-driven small molecule generation platform, Chemistry42. Employing structure-based drug design (SBDD) strategies and employing stringent filters for similarity and selectivity, the research team successfully crafted a diverse array of compounds meticulously tailored to target MYT1.
Among these novel compounds, a specific series emerged as highly promising candidates. The research team conducted an intensive X-ray crystal structure analysis of a complex, unearthing the impact of subtle chemical modifications on compound activity. Armed with this invaluable knowledge, further molecular optimization endeavors ensued, ultimately culminating in the discovery of the lead compound, aptly named Compound 21.
The Exceptional Potential of Compound 21
Compound 21 exhibits notable MYT1 activity coupled with outstanding selectivity over Wee1 and other kinases, thereby minimizing the risk of off-target effects. This remarkable attribute contributes to its potential as a safe and efficacious therapeutic agent. Preclinical studies have demonstrated Compound 21’s substantial in vivo antitumor efficacy, highlighting its promising profile in ADME (absorption, distribution, metabolism, and elimination) and PK/PD (pharmacokinetic/pharmacodynamic) studies.
A Novel Therapeutic Outlook
“The innovative approach of this program has not only presented a method for effective target identification but has also led to the development of a promising selective MYT1 inhibitor,” remarked Dr. Yazhou Wang, the medicinal chemistry leader of the MYT1 program at Insilico Medicine and the first author of the published paper. This groundbreaking study harnesses the power of AI and advanced computational techniques to unlock the therapeutic potential of MYT1 in breast and gynecological cancers, offering a glimmer of hope to countless women affected by these devastating diseases.
The study serves as a testament to the transformative potential of AI-driven research in modern medicine. By shedding light on MYT1’s significance and unveiling a series of highly selective inhibitors, this research paves the way for the development of innovative therapies that can make a profound impact on the lives of cancer patients. The future holds great promise as we continue to explore the intersections of AI, biology, and chemistry, revolutionizing the field of oncology and the quest for effective cancer treatments.

Leave a Reply