As the use of electric and hybrid vehicles continues to grow globally, the demand for safe and high-performing battery technologies becomes increasingly crucial. Engineers and researchers are actively working on improving battery safety, energy capacity, scalability, and degradation over time. One promising area of focus is rechargeable multivalent metal batteries, specifically those utilizing low-reduction potential anode materials like magnesium (Mg) and calcium (Ca). These batteries have the potential for high energy densities if developed using the right combination of anodes, cathodes, and electrolytes.
New Method for Highly Performing and Scalable Electrolytes
Recently, a team of researchers from Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology introduced a groundbreaking method for producing highly performing and scalable electrolytes for multivalent metal batteries. Their innovative strategy, outlined in a paper published in Nature Energy, offers a promising solution for developing reversible and cost-efficient electrolyte systems that could revolutionize next-generation battery technologies.
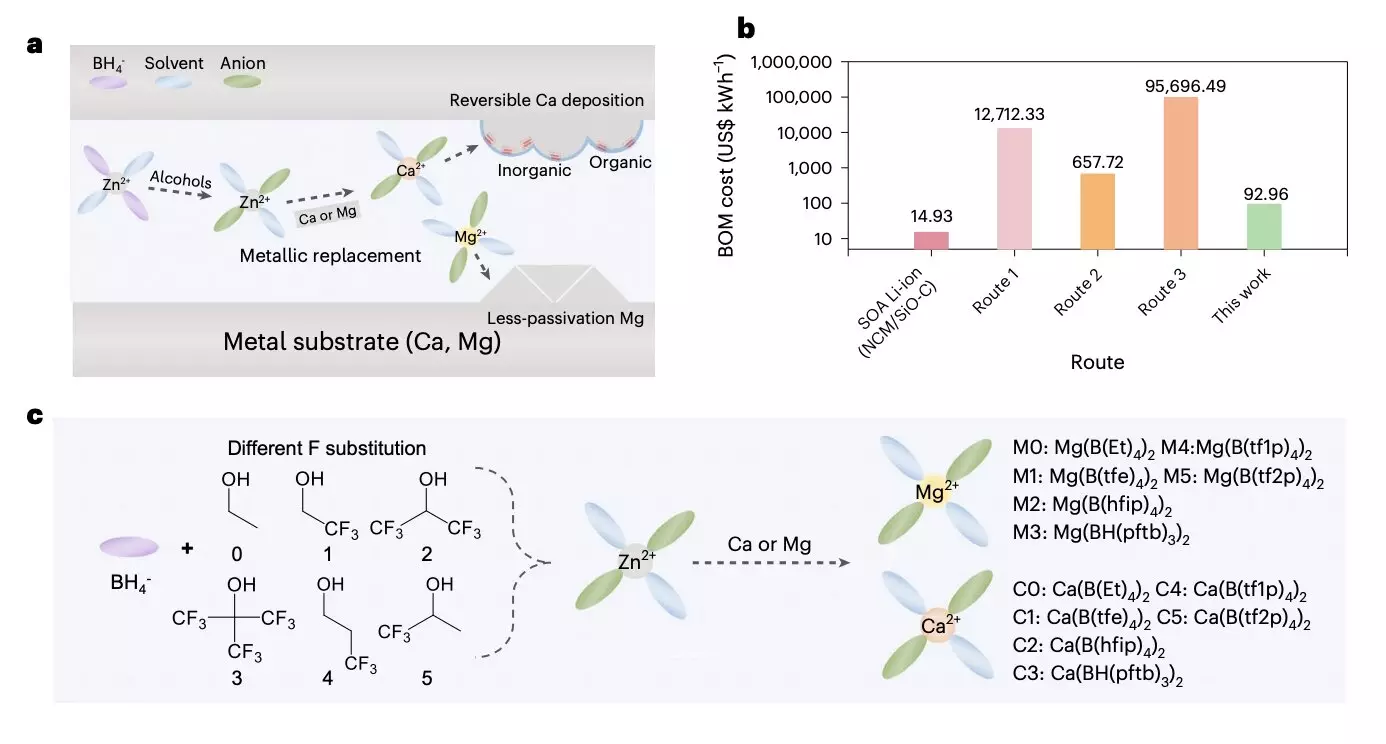
In their study, the researchers developed a universal cation replacement method to prepare low-cost, high-reversibility magnesium and calcium electrolytes derived from a zinc organoborate solvation structure. The process involved several steps, including the chemical reaction between an affordable and easily obtainable Zn(BH4)2 precursor and different fluoroalcohols, resulting in the production of target anions with various branched chains. Subsequently, these anion solvates reacted with low-cost metal foils with higher metal activity, generating target solvation structures. To ensure stable battery cycling, the researchers proposed the formation of a passivation layer based on two types of calcium solvates, which effectively suppressed solvent decomposition.
Promising Results and Future Implications
The method developed by the research team has already yielded promising results. They successfully created a high-loading battery prototype based on Mg/S, with a capacity of 53.4 Wh/kg. The prototype featured a 30 μm Mg anode, a low electrolyte/sulfur ratio (E/S = 5.58 μl/mg), and a modified separator/interlayer. Initial tests demonstrated the potential of this approach, highlighting the possibility of producing favorable and low-cost electrolytes for multivalent metal batteries.
Looking ahead, the method introduced in this study holds significant potential for the future development of various reversible electrolyte systems. By utilizing more affordable materials and simpler processing strategies, these electrolytes could pave the way for the creation of scalable and safe multivalent metal batteries with higher energy densities.
The ongoing advancements in battery technology are crucial to support the growing demand for electric and hybrid vehicles. The development of safe, high-performing, and scalable battery technologies remains a top priority for engineers and researchers worldwide. The recent introduction of a new method for producing highly performing and scalable electrolytes for multivalent metal batteries signifies a significant breakthrough in this field. The research team’s universal cation replacement method offers a cost-effective and reversible solution that could revolutionize the future of battery technologies. With further research and development, these electrolytes have the potential to enable the creation of next-generation batteries with improved energy densities, contributing to a cleaner and more sustainable future.

Leave a Reply