Ribosomes are essential in the process of protein synthesis, acting as the cell’s “protein factories.” They consist of two subunits, with the small subunit responsible for reading the messenger RNA (mRNA) and the large subunit responsible for protein synthesis. While the assembly of ribosomes is well-understood, the degradation process has remained a mystery. However, a research team from the Universität Hamburg has now made a groundbreaking discovery, identifying the dynamic mechanism used by the enzyme RNase R to degrade the ribosomal 30S subunit.
In times of stress or the end of the cell’s growth cycle, the stationary phase is entered, where cells reduce their metabolism to survive longer. As a part of this process, some ribosomes are degraded to release the energy invested in them. Until now, the structural insight into ribosome degradation has been lacking, hindering our understanding of this crucial cellular process.
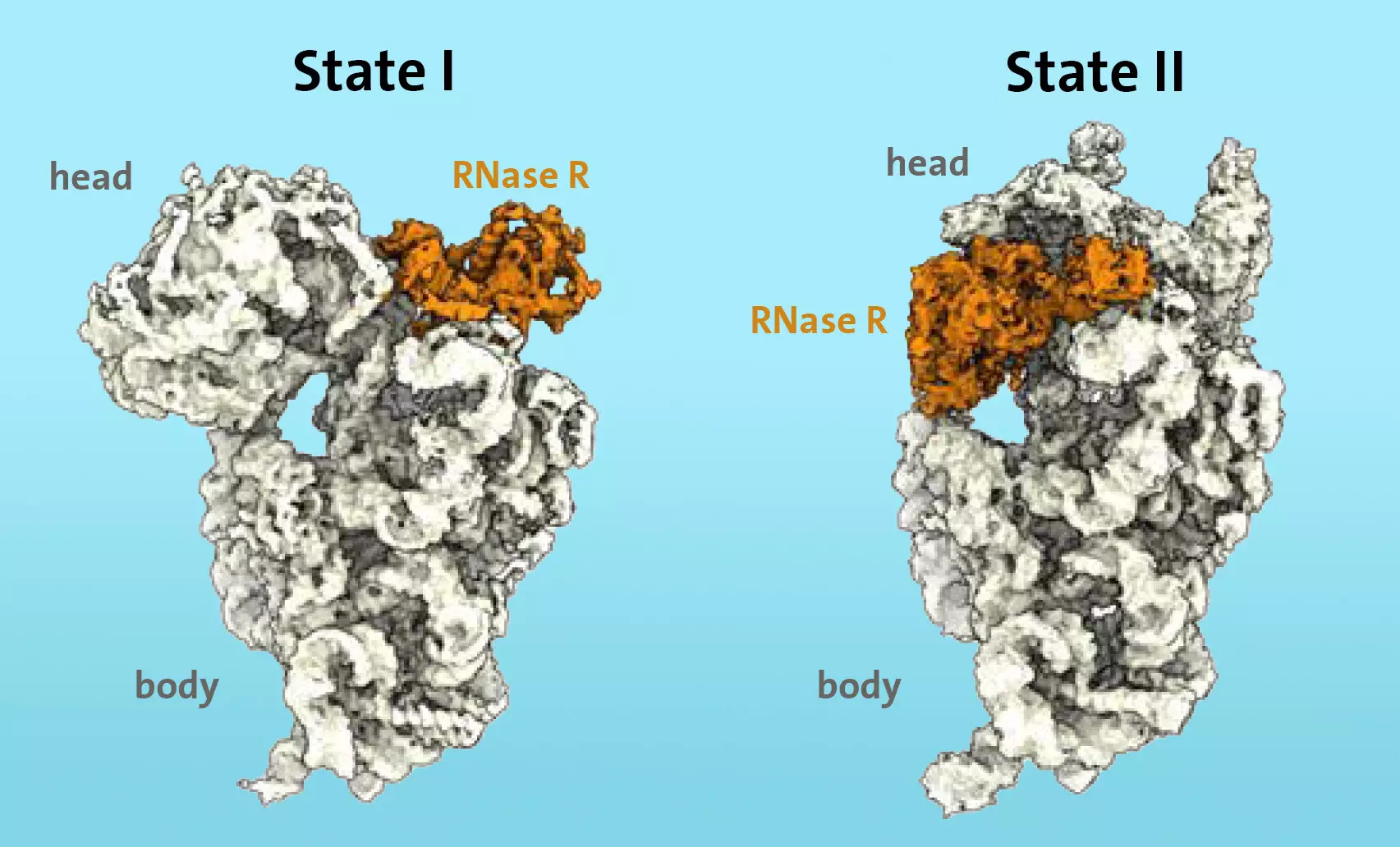
The researchers at the Universität Hamburg focused their study on Bacillus subtilis, a common soil bacterium found in various environments. They specifically looked at cells that were still growing, aiming to understand the processes occurring at the transition to the stationary phase. Previous research had identified RNase R as an enzyme involved in ribosome degradation in stress situations. Using cryo-electron microscopy, the team was able to observe the binding and action of RNase R on the ribosomal 30S subunit.
Through their experiments, the researchers discovered that RNase R does not randomly cut the 30S subunit but rather binds to a specific area known as the “neck.” The enzyme then detaches the “head” of the subunit in two stages. First, RNase R destabilizes the neck area, making it more flexible. In the second stage, the enzyme turns the head, removing the obstacle and allowing for the unhindered degradation of the 30S subunit to continue.
The researchers found that the “head” switch in the degradation process is a significant kinetic barrier for RNase R. It acts as a crucial checkpoint that controls the rate of degradation. Additionally, the experiments showed that the enzyme RNase R alone is sufficient to accomplish the complete 30S degradation process.
The identification of the dynamic mechanism used by RNase R to degrade the ribosomal 30S subunit provides crucial insights into the degradation of ribosomes, a process that plays a vital role in cell survival during stress conditions. This newfound understanding opens up possibilities for further research into the regulation of ribosome degradation and its implications in cellular processes. Additionally, the study adds to our knowledge of the complex machinery of protein synthesis and the intricacies of ribosomal function.
The research conducted by the Universität Hamburg’s Department of Chemistry sheds light on a previously unknown process in cellular biology: the degradation of ribosomes. By identifying the dynamic mechanism used by the enzyme RNase R to degrade the ribosomal 30S subunit, the study significantly contributes to our understanding of cellular survival strategies and the regulation of protein synthesis. As further investigations build upon these findings, deeper insights into the molecular mechanisms at work in the cell will continue to emerge, paving the way for potential advancements in medicine and biotechnology.

Leave a Reply