In a groundbreaking discovery, scientists at RIKEN have successfully isolated a mysterious structure involving two water molecules that had been predicted but never observed. This significant breakthrough could have far-reaching implications for various fields such as astrochemistry and the study of metal corrosion. The research paper detailing this finding is published in The Journal of Physical Chemistry Letters.
When an energetic particle or a photon interacts with a water molecule, it can result in the ejection of an electron, leading to the formation of a positively charged ion (cation; H2O+) and a free electron. This ionization process sets off a series of subsequent reactions with adjacent molecules. Understanding the ionization of water is crucial for comprehending biological processes, radiation chemistry, and the corrosion of metals at water-metal interfaces.
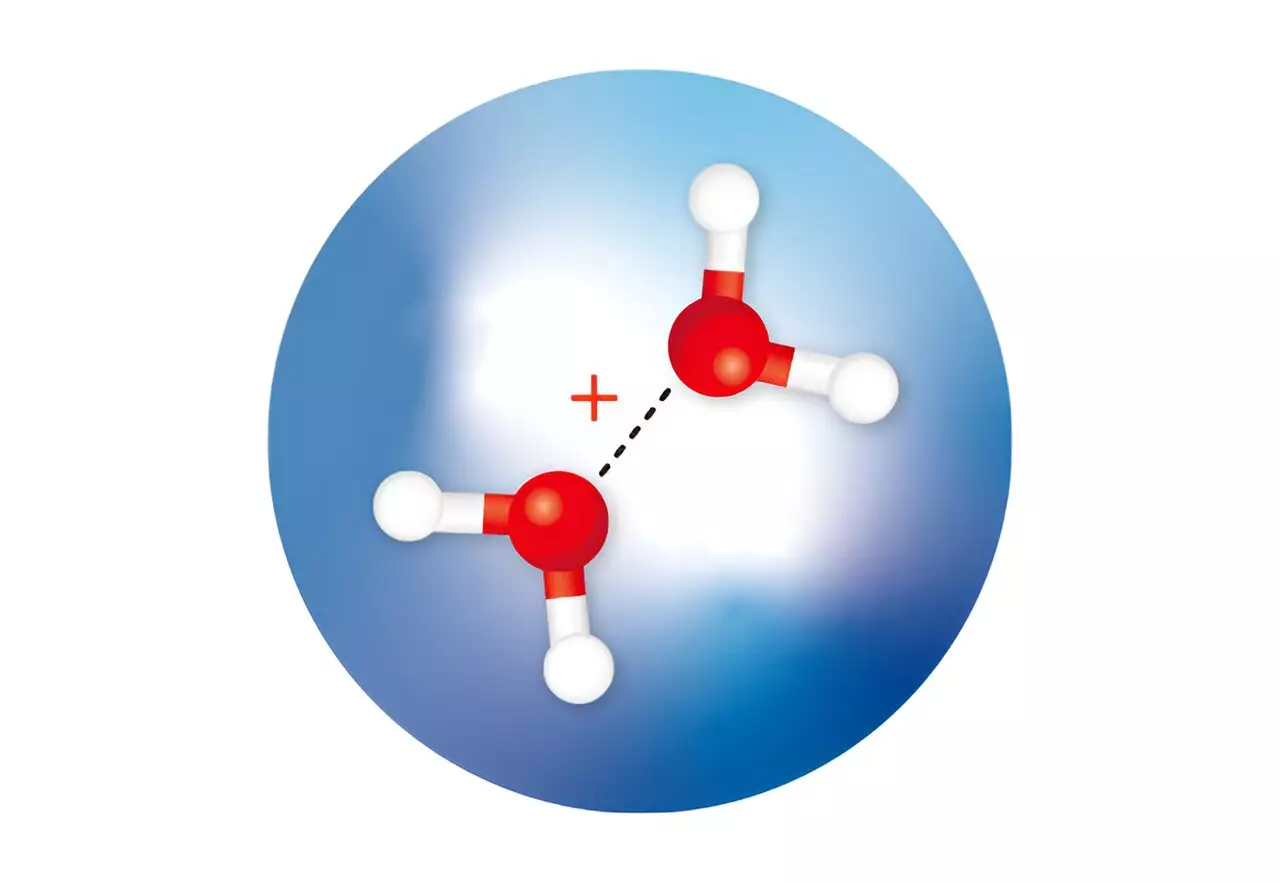
Theoretical calculations have long suggested that after the ionization of a water molecule, two isomers of a positively charged ion, known as a water dimer, are rapidly formed. One isomer, called H3O+·OH, has been observed and occurs when a proton is transferred from one water molecule to another. The other isomer, referred to as (H2O·OH2)+, has a half-bonded or hemibonded structure but has never been isolated or confirmed through spectroscopic measurements. Calculations indicate that this isomer possesses higher energy compared to the proton-transfer dimer.
Susumu Kuma and his team from the RIKEN Atomic, Molecular, and Optical Physics Laboratory have successfully isolated both water dimer ions by trapping them in minuscule droplets of cold helium. Additionally, they employed infrared spectroscopy to determine the structures of these elusive ions. The researchers created an ultracold environment, causing the water molecules in the helium droplets to rapidly cool as helium atoms evaporated from the surface of the droplets. This cooling process facilitated the formation of the metastable hemibonded isomer within the cold droplets due to its rapid stabilization.
Kuma and his colleagues used computational and spectroscopic methods to examine the co-existence of the two isomers. The spectroscopic signatures of these molecular ions closely resembled those of bare ions, indicating that direct comparisons can be made between measurements on the bare ions and results from quantum-chemical calculations. This breakthrough enables more precise structural analyses of the water dimer ions.
Susumu Kuma predicts that this discovery of hemibonded water cations will initiate further studies into the primary events necessary for comprehending the radiation chemistry of water. With this newfound knowledge, researchers can delve deeper into the intricate mechanisms underlying water’s behavior and explore other yet-to-be-observed structures. Kuma and his team have plans to expand their investigations by studying larger water complex cations within helium droplets.
The isolation and characterization of the elusive water dimer ions by RIKEN chemists represent a significant advancement in our understanding of the ionization of water and its subsequent reactions. This discovery opens up avenues for research in various fields, such as radiation chemistry and the study of primary events crucial for unraveling the mysteries of water behavior. By shedding light on the hidden link between water molecules, the possibilities for future discoveries and scientific breakthroughs are boundless.

Leave a Reply