The process of converting carbon dioxide into valuable chemicals has recently gained significant attention due to the potential to mitigate the impacts of greenhouse gases on the environment. A collaborative project involving the U.S. Department of Energy’s Argonne National Laboratory, Northern Illinois University, and Valparaiso University has made a breakthrough in this area by developing a family of catalysts based on tin metal that can efficiently convert CO2 into ethanol, acetic acid, and formic acid.
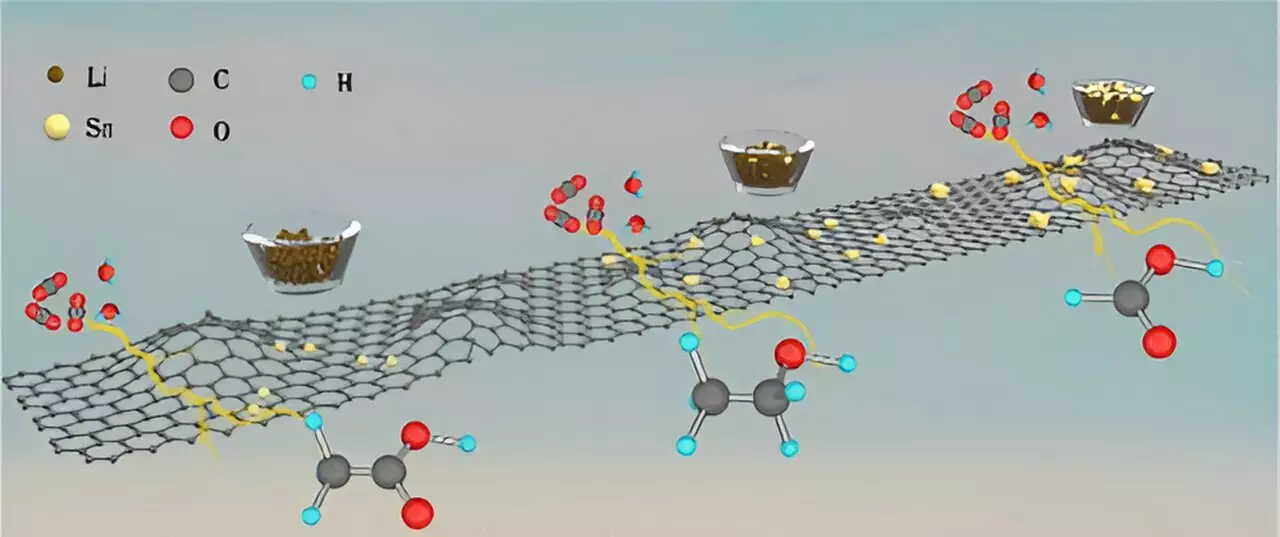
The catalysts developed by the research team are based on tin metal deposited over a carbon support. This unique design allows for the selective conversion of CO2 into different chemicals depending on the size of tin used in the catalyst. By varying the tin size from single atoms to larger nano-crystallites, the team was able to achieve high selectivity for ethanol, acetic acid, and formic acid, with each chemical reaching a selectivity of 90% or higher.
Insights into Reaction Mechanisms
Through computational and experimental studies, the research team gained valuable insights into the reaction mechanisms involved in the conversion of CO2 into liquid hydrocarbons. One significant discovery was the changing reaction path based on the catalyst size, which was previously unprecedented. Additionally, the team observed the kinetic isotope effect when switching from using ordinary water to deuterated water in the conversion process, highlighting the complexity of the chemical reactions involved.
Utilizing Advanced Facilities
The success of this research project was greatly aided by the use of advanced facilities at the Argonne National Laboratory, including the Advanced Photon Source (APS) and the Center for Nanoscale Materials (CNM). These facilities provided the necessary tools to analyze the chemical and electronic structures of the tin-based catalysts with high precision, leading to a better understanding of their performance in CO2 conversion.
The ultimate goal of the research team is to integrate the newly discovered catalysts into low-temperature electrolyzers powered by renewable energy sources such as wind and solar. This approach would enable the on-site production of valuable chemicals from CO2, reducing the need for expensive transport and storage of carbon dioxide. By utilizing locally generated electricity, the team aims to create a sustainable and efficient process for converting greenhouse gases into useful products.
The development of tin-based catalysts for the conversion of carbon dioxide represents a significant step towards achieving sustainable chemical production processes. By leveraging the unique properties of tin and utilizing cutting-edge research facilities, the team has demonstrated the potential to transform greenhouse gases into valuable chemicals with high selectivity and efficiency. This research paves the way for future advancements in the field of carbon dioxide utilization and opens up new possibilities for reducing the environmental impact of industrial operations.

Leave a Reply