Proteins, the molecular machines essential for cellular functions, rely on their three-dimensional structures for proper functioning. Recent advancements in protein research have led to the development of a groundbreaking mathematical method, known as LoCoHD (Local Composition Hellinger Distance), by the HUN-REN-ELTE Protein Modeling Research Group. Unlike traditional methods that only consider atom positions, LoCoHD incorporates crucial chemical information of atoms, revolutionizing protein structure comparison.
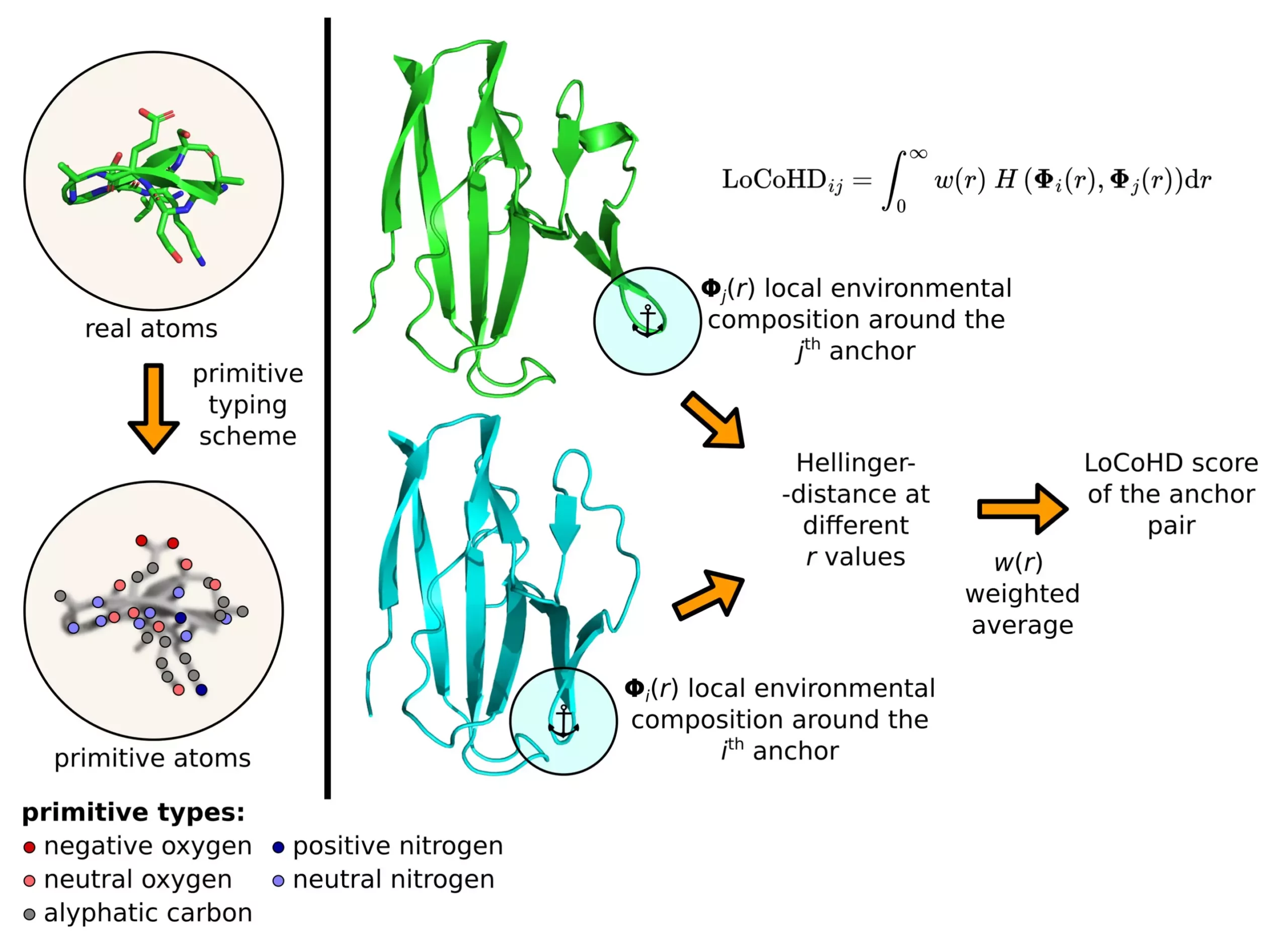
Developed by Zsolt Fazekas and Dr. András Perczel’s research group, the LoCoHD algorithm assesses local environments around amino acids based on their chemical properties. By assigning a numerical value ranging from 0 to 1 to indicate structural differences, the method provides valuable insights into protein systems. Through a multi-step protocol, LoCoHD converts real atoms into primitive atoms, allowing for detailed comparisons of atomic arrangements and chemical properties.
The versatility of the LoCoHD method extends to various aspects of protein research, including participation in the prestigious CASP (Critical Assessment of Protein Structure Prediction) competitions. By incorporating chemistry into structure comparisons, researchers gain a deeper understanding of protein dynamics and interactions. Notably, the analysis of the SARS-CoV-2 virus protein ORF8 and the HIV-1 capsid protein has revealed critical insights that could advance drug development and therapeutic strategies for severe diseases.
In addition to static structure analysis, LoCoHD proves effective in studying the internal motion of proteins. By analyzing molecular motions and structural ensembles, researchers can identify key amino acids undergoing significant chemical changes during protein movement. This capability has profound implications for studying proteins essential for vital functions, such as the podocin protein in the kidney, offering potential insights into severe, life-threatening conditions caused by protein mutations.
The groundbreaking applications of the LoCoHD method in protein research pave the way for enhanced drug development and therapeutic interventions. By enabling researchers to dissect intricate protein structures and interactions, LoCoHD facilitates a comprehensive understanding of disease-causing pathogens. Through targeted studies on key proteins, such as those involved in viral replication and pathogenesis, researchers can advance the development of novel treatments and interventions for a wide range of diseases.
The emergence of the LoCoHD method marks a significant step forward in protein structure analysis and computational modeling. By integrating chemical information into structural comparisons, researchers can uncover novel insights into protein dynamics, interactions, and functional mechanisms. The applications of LoCoHD in studying dynamic protein structures and disease-related proteins offer promising prospects for advancing drug discovery efforts and therapeutic strategies. As researchers continue to harness the power of computational methods, we move closer to unraveling the complexities of protein biology and developing innovative solutions to combat challenging diseases.

Leave a Reply