In a groundbreaking development, a team of researchers led by Professor Han Gi Chae and Professor Jong-Beom Baek at UNIST, in collaboration with Professor Kafer T. Tavuz at KAUST, has introduced a new technology to overcome the limitations of current catalyst electrodes. The innovation, reported in the Journal of the American Chemical Society, involves the creation of carbon fabric electrocatalysts embedded with highly functional catalysts using a unique manufacturing process. This advancement allows for the production of green hydrogen on a large scale and at a much lower cost compared to traditional electrodes.
Traditionally, electrochemical electrodes were manufactured by applying a powder catalyst onto the electrode, which often led to issues such as uneven application, clumping, and detachment. This outdated method hindered the stability and efficiency of the electrodes, limiting their lifespan and overall performance. The research team recognized the need for a new approach to address these challenges and enhance the effectiveness of catalyst electrodes.
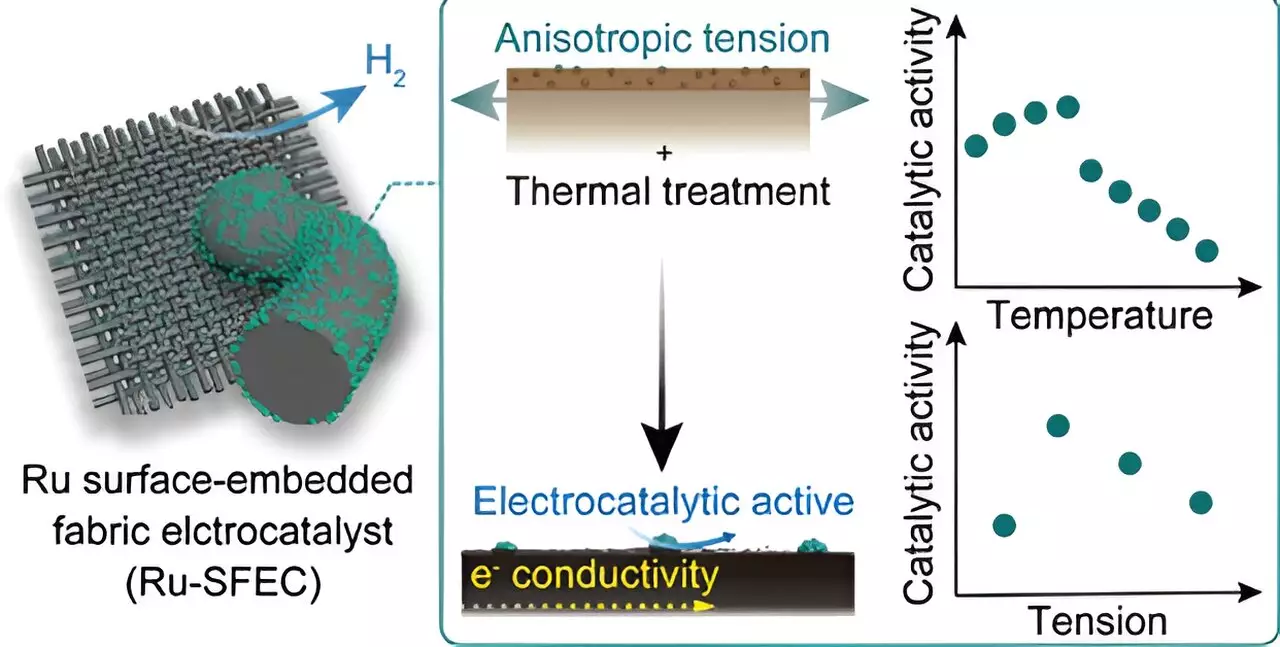
The team’s innovative design involves integrating ruthenium into the carbon fabric electrode during the manufacturing process, resulting in the creation of ruthenium surface-embedded fabric electrocatalysts (Ru-SFECs). This design boasts a lifespan 100 times longer than conventional electrodes and exhibits optimal performance by utilizing ruthenium instead of the more expensive platinum. Notably, the Ru-SFECs demonstrate low overvoltage and energy consumption during the hydrogen generation process, showcasing remarkable stability and efficiency.
Carbon fiber-based electrochemical electrodes have gained attention for their high thermal and electrical conductivity properties, making them an ideal choice for large-scale applications. By incorporating ruthenium into the polymer precursor fiber early in the manufacturing process, the team was able to achieve maximum stability and activity. This approach not only reduces manufacturing costs but also leads to a significant cost advantage compared to traditional platinum-based electrodes.
The team’s innovative approach leverages the exceptional mechanical and electrical properties of carbon fibers, opening up new possibilities for future electrochemical reactions. By meticulously controlling catalyst metal separation and microcarbon structure, the researchers have paved the way for the continuous production of catalyst fibers for direct industrial applications. This breakthrough lays a foundation for developing stable, binder-free, and flexible electrocatalytic electrodes with the potential for various catalytic reactions involving different metals.
The development of carbon fabric electrodes embedded with highly functional catalysts represents a significant advancement in the field of electrochemical electrodes. The innovative design not only offers energy-efficient manufacturing processes but also reduces waste production. The team’s groundbreaking research paves the way for the widespread adoption of carbon fiber electrodes in various industrial applications, showcasing their potential as a versatile material for future electrochemical reactions. Further research should focus on enhancing mechanical durability, electrical conductivity, and cost-effectiveness to fully realize the benefits of this revolutionary technology.

Leave a Reply