Hydrogen (H2) has shown promise as a fuel that can help reduce greenhouse gases when produced through water splitting using renewable energy. However, the process of breaking water into hydrogen and oxygen is not as straightforward as it may seem. It involves complex chemistry with two simultaneous electrochemical reactions requiring catalysts to facilitate the breaking and remaking of chemical bonds.
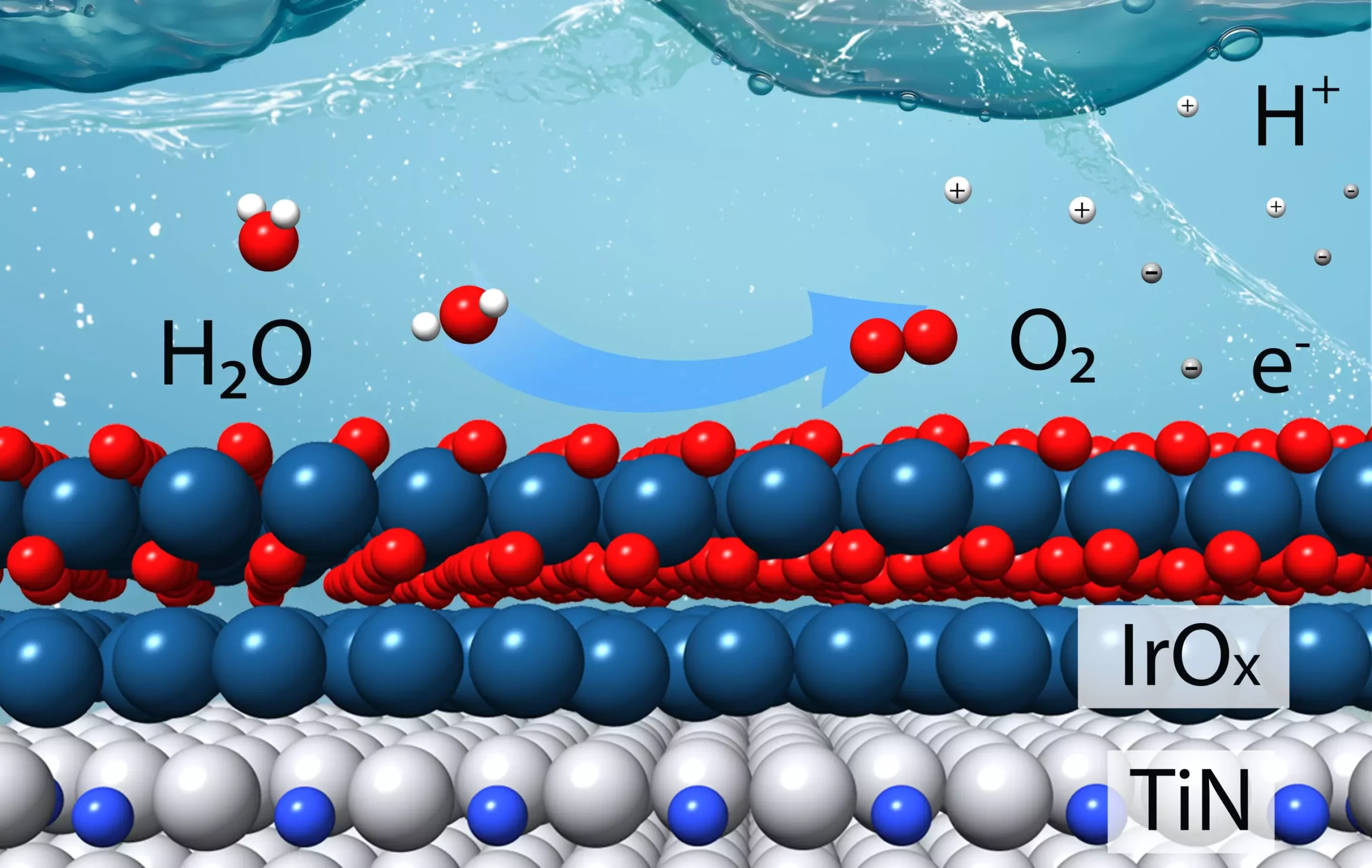
Researchers at the U.S. Department of Energy’s Brookhaven National Laboratory and Columbia University have recently developed a new efficient catalyst designed specifically for the challenging oxygen evolution reaction. This catalyst was meticulously crafted based on theoretical calculations aimed at minimizing the use of iridium, an expensive metal commonly used in catalytic materials, while maximizing stability in acidic conditions.
The new catalyst, described in a paper published in the Journal of the American Chemical Society, has shown remarkable efficiency in producing hydrogen in a water-splitting electrolyzer. In fact, it outperforms the current state-of-the-art commercially available iridium catalyst by a factor of four. This means that the new catalyst requires four times less iridium to produce hydrogen at the same rate or produces hydrogen four times faster for a given amount of iridium.
The development of this innovative catalyst was driven by theoretical calculations conducted by Brookhaven Lab’s theoretical chemist Ping Liu. These calculations provided insights into how different overlayers of iridium on titanium nitride could impact the catalyst’s stability and activity under acidic oxygen evolution reaction conditions.
The researchers not only created thin films with carefully controlled layers that mimicked the surfaces used in the theoretical modeling but also experimented with powdered samples composed of small nanoscale particles, mirroring the catalyst’s form in industrial applications. Characterization studies using transmission electron microscopy and X-ray spectroscopy confirmed the effectiveness of the catalyst in enhancing hydrogen production.
Although the new catalyst has demonstrated impressive performance in the lab, the researchers acknowledge that scaling up production remains a significant challenge. While the catalyst shows great promise in laboratory settings, making it applicable to industrial-scale production requires further optimization.
The development of this highly efficient catalyst marks a significant step forward in the quest for green hydrogen production. By reducing the reliance on expensive and rare elements like iridium, this catalyst has the potential to lower the cost of water splitting and accelerate the transition towards sustainable energy sources. Further research and development are needed to address the scalability of production and optimize the catalyst for real-world applications.

Leave a Reply