In the realm of chemistry, the pursuit of chiral molecules is fundamental to various applications, from pharmaceuticals to materials science. Chiral molecules, which cannot be superimposed on their mirror images, play a crucial role in the effectiveness of many biological processes. Traditionally, enzymes, natural biological catalysts, have been employed to synthesize these molecules due to their effectiveness. However, the stability of these proteins can be a significant challenge, leading researchers to explore alternative catalysts, particularly DNA.
The recent advancements by chemists at the National University of Singapore (NUS) represent a significant stride towards utilizing DNA as a chiral scaffold for asymmetric catalysis. By merging biorthogonal chemistry with DNA repair processes, the NUS team has forged a pathway for the efficient production of diverse chiral DNA catalysts. These catalysts offer several advantages over traditional enzymatic methods, primarily their stability and programmability. Since DNA consists of stable molecular structures, it allows for the creation of catalysts that can withstand various catalytic conditions without the frequent degradation that enzymes experience.
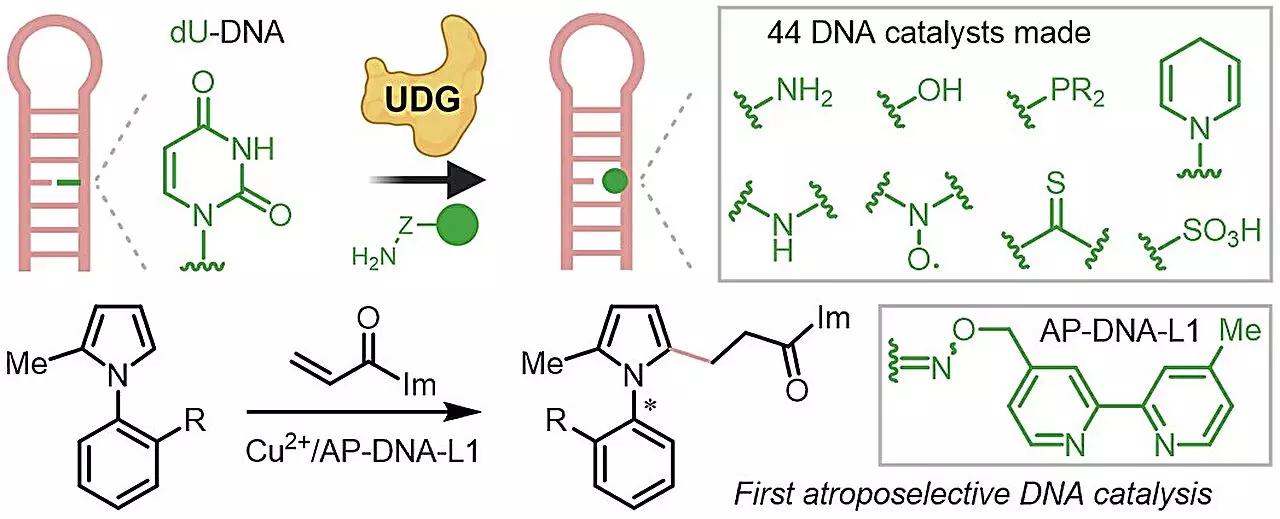
Methodology: DNA Repair Meets Biorthogonal Chemistry
Under the guidance of Assistant Professor Zhu Ru-Yi, the NUS researchers innovatively utilized an enzymatic mechanism known for its ability to repair DNA. By employing biorthogonal reactions, which occur independently of biological processes, the researchers simplified the complex task of creating DNA catalysts. This groundbreaking approach lowers the entry barrier for those interested in this field, enabling non-specialists to perform sophisticated DNA catalysis without high-end resources or extensive training. The versatility of the biorthogonal reactions enhances the compatibility of these novel catalysts with various functional groups, further broadening their applicability in real-world scenarios.
The research unveiled a repertoire of 44 new DNA catalysts, significantly enhancing the efficiency, enantioselectivity, and substrate scope compared to existing alternatives. One of the standout achievements of this study was the demonstration of atroposelective DNA catalysis, a process known for its complexity, allowing for the successful synthesis of axially chiral compounds that pose challenges in conventional bio-catalysis. This accomplishment not only underscores the potential of DNA as a reliable catalyst but also opens avenues for developing new synthetic strategies that were previously not feasible.
Future Directions in DNA Catalysis
As the NUS team continues to refine their techniques, the future looks promising for DNA catalysis in sustainable chemistry. The ongoing exploration aims to enhance the selectivity and efficiency of chemical reactions further. By leveraging the unique properties of DNA and biorthogonal chemistry, researchers could potentially revolutionize the way asymmetric catalysis is approached, leading to sustainable practices that align with environmentally friendly chemical production.
The innovative research from NUS exemplifies the evolving landscape of asymmetric catalysis, suggesting compelling prospects for future applications within both academia and industry. With continued exploration and refinement, the integration of DNA catalysis into mainstream chemistry could significantly boost the development of chiral compounds while adhering to sustainability principles.

Leave a Reply