In recent years, the fields of physics and biochemistry have increasingly overlapped, particularly in the study of complex biological systems. At São Paulo State University (UNESP), researchers have ventured into this interdisciplinary territory by applying concepts from condensed matter physics—specifically, Griffiths-like phase behavior—to understand protein compartmentalization within cells. This innovative approach provides new insights into the dynamics of cellular processes, potentially elucidating pathways related to the origins of life and the mechanisms of various diseases. The foundational principles of mixture theory serve as a backdrop for this research, highlighting the intricate interactions and compartmentalization that can arise in biological systems.
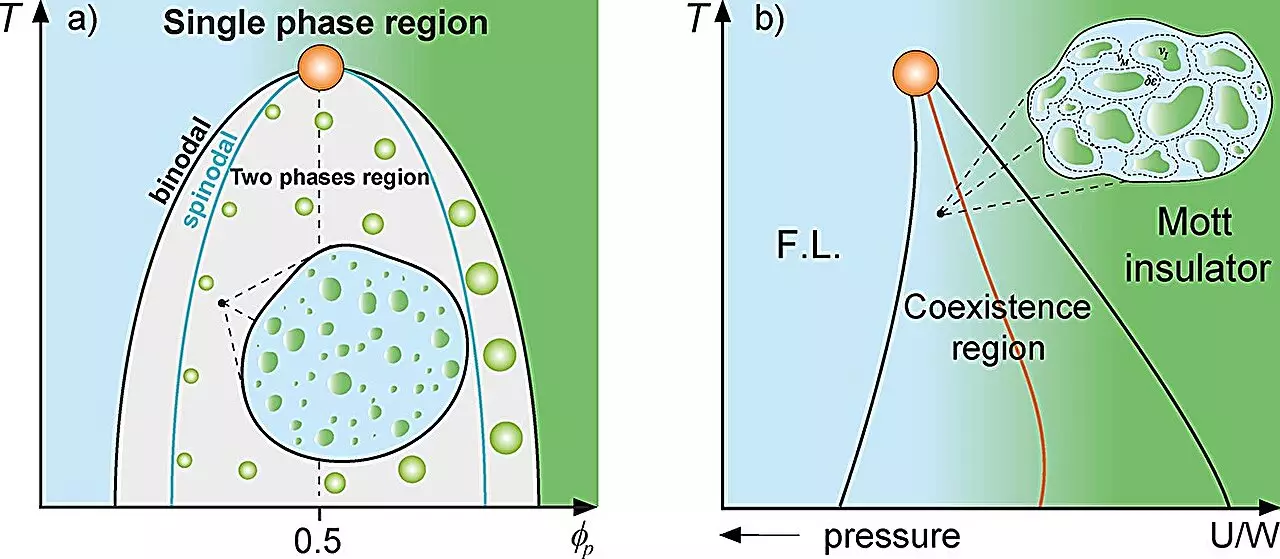
Griffiths phases are a phenomenon observed in disordered systems, manifesting as distinct regions where particles exhibit varying magnetic properties—some may be magnetized, while others may not, all within the same medium. This concept is pivotal in understanding how certain configurations can influence system dynamics. The research team at UNESP leveraged the paradigm of the Griffiths phase to frame their observations of protein droplets formed through liquid-liquid phase separation within cellular environments. These “rare regions,” similar to those noted in magnetic Griffiths models, suggest that certain dynamics in biological systems are fundamentally altered at specific thresholds of protein concentration, echoing observations from earlier studies in condensed matter physics.
In their study, the team utilized a variety of thermodynamic tools—including the Grüneisen parameter and models such as Flory-Huggins—to investigate protein behaviors and relationships within cells. Their findings indicate a significant slowdown in cellular dynamics near the phase separation boundary, a phenomenon reminiscent of what might occur in physical systems undergoing similar transitions. By mapping these interactions and conditions under which proteins coalesce into droplets, the researchers propose an analogy to Griffiths-like behavior, contributing to a deeper understanding of cellular organization and function.
The implications of such protein compartmentalization stretch beyond mere structural organization; they suggest a link to life’s origin. Drawing from the pioneering theories of Russian biochemist Aleksandr Oparin, the researchers propose that ancestral forms of life may have relied on these slow-moving coacervates—organic molecule clusters that may have been critical for survival and evolution in primordial environments.
One particularly intriguing aspect of the UNESP team’s findings is the role of chirality in cellular systems. Chirality, a property indicating that certain molecules are non-superimposable on their mirror images, emerges as a significant factor in biological processes where homochirality—the dominance of a single chiral form—is prevalent. Understanding how protein dynamics and droplet formation relate to homochirality could be pivotal in studying the biochemical pathways underpinning life itself.
The study’s conclusions indicate a correlation between increased protein diffusion times and reduced stochastic fluctuations in cellular environments. These dynamics are crucial in optimizing gene expression, suggesting that phase behavior can directly influence biological outcomes. Thus, not only does the research expand theoretical knowledge, but it also highlights potential practical applications in biotechnology and synthetic biology.
The relevance of liquid-liquid phase separation extends into medical contexts, particularly concerning disease mechanisms. The team emphasizes that proteins linked to various diseases, such as tumors or neurodegenerative conditions, may be affected by their compartmentalization, thereby influencing their functional roles in cellular mutations. Interestingly, the potential impact of Griffiths-like phases opens avenues for therapeutic strategies, providing a foundation for further studies on how manipulating protein behaviors might mitigate disease progression or even foster healing.
For instance, the ongoing discourse surrounding conditions like cancer and COVID-19 illustrates how phase separation phenomena can be either detrimental or beneficial, altering the dynamics of disease. Following this trajectory, the researchers encourage exploration into the regulatory mechanisms underlying these phenomena to better understand their contributions to health and disease.
The collaborative nature of this research underscores the importance of interdisciplinary efforts in modern science. By bridging the gap between physics and biology, the UNESP team not only enhances our understanding of fundamental cellular processes but also sets the stage for innovative applications in medicine. Future research may expand on this Griffiths-like cellular phase concept, advancing both theoretical models and practical investigations into protein dynamics.
Overall, the work elucidates a fascinating interplay between the physical structure of proteins, their behavior under particular conditions, and the profound implications these dynamics hold for the evolution of life and the understanding of disease mechanisms. As we continue to unravel the complexities of cellular processes, the fusion of physics and biology will undoubtedly yield transformative insights for both scientific disciplines.

Leave a Reply