Recent findings from scientists at Oak Ridge National Laboratory (ORNL) have unveiled crucial insights into the functioning of serine hydroxymethyltransferase (SHMT), an enzyme that plays a pivotal role in the metabolic landscape of cancer cells. With the aid of advanced neutron experiments conducted at the Spallation Neutron Source and the High Flux Isotope Reactor, researchers have been able to explore the enzyme’s atomic-scale chemistry in unprecedented detail. This breakthrough not only enhances our understanding of cancer metabolism but also holds the potential for innovative drug design aimed at aggressive cancers.
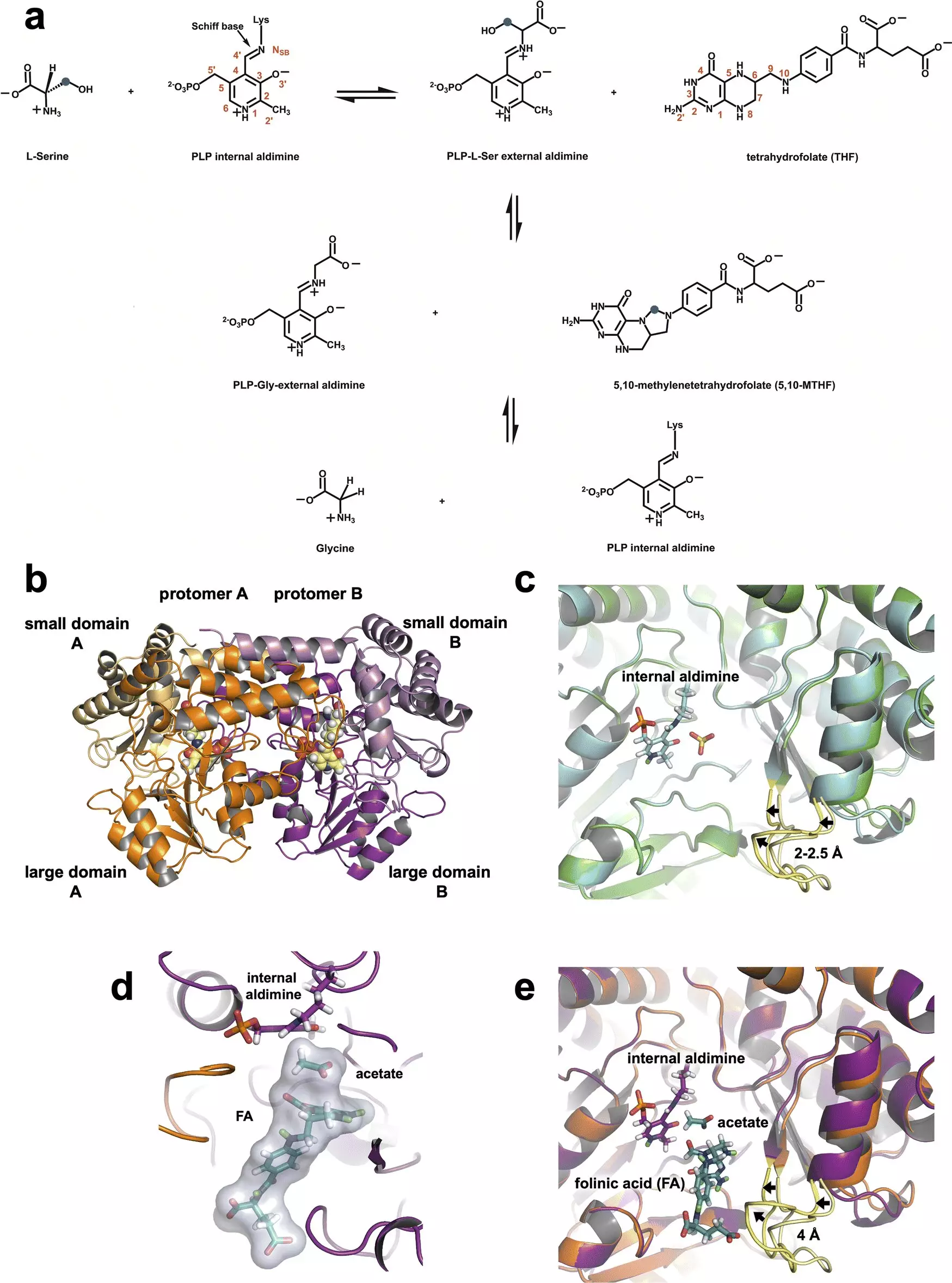
SHMT is a metabolic enzyme integral to cell division, as it catalyzes the conversion of serine to glycine, linking to the one-carbon metabolic pathway essential for the synthesis of critical biological molecules, including DNA and RNA. In cancer, however, SHMT’s function can spiral out of control, causing rapid reproduction of cancer cells. By designing inhibitors that target this enzyme effectively, scientists hope to interrupt the cancer progression by accessing this early stage of the metabolic pathway.
The importance of neutron diffraction in this research cannot be overstated. Neutrons are particularly adept at revealing the positions of light atoms, such as hydrogen, which are crucial to understanding the specific catalytic mechanisms of enzymes. In this case, researchers discovered that a glutamate residue within SHMT holds significant responsibility for regulating its catalytic actions. This detailed observation has the potential to inform targeted drug design, which could provide a new avenue for treatment.
Dr. Victoria Drago of ORNL noted, “Neutrons allow us to see hydrogen atoms, and hydrogen drives chemistry.” This ability to visualize the positioning of hydrogen not only clarifies the enzyme’s electrostatic environment but also assists in understanding the enzyme’s behavior under physiological conditions. The knowledge gleaned from such studies enables researchers to identify how inhibitors might effectively disrupt SHMT’s activity.
In their investigations, the team employed a multifaceted approach by integrating neutron diffraction with X-ray crystallography, a technique that examines heavier elements such as carbon and nitrogen. By capturing the dynamics of SHMT at room temperature during its interaction with tetrahydrofolate, researchers established certainty in their understanding of the enzyme’s reaction mechanisms, building upon decades of prior studies that remained inconclusive.
The combination of these techniques presents a significant advantage, allowing researchers to visualize the reactive sites within the enzyme more clearly. By identifying the precise location and behavior of hydrogen atoms, scientists can better understand the electrostatics involved in the enzyme’s catalytic function, crucial knowledge for designing new small-molecule inhibitors that would effectively target SHMT.
The findings highlight the potential of SHMT as an early target in the one-carbon metabolic pathway, setting it apart from other components that are already established targets for cancer treatments. Conventional cancer therapies often exhibit limited success due to the cancer cells’ ability to bypass targeted mechanisms and reconfigure their metabolic processes. Therefore, interrupting SHMT’s role at the onset of this complex metabolic train could yield more effective outcomes.
By advancing our comprehension of SHMT’s functionality, researchers could develop more sophisticated therapies with the aim of combating cancer before it has a chance to metastasize. Dr. Andrey Kovalevsky emphasized that understanding the atomic details of SHMT “can inform the design of an inhibitor to target this specific protein as part of a combination therapy.” The goal is not only to inhibit cancerous activity but to do so with minimal adverse effects on healthy cells—a significant hurdle in the field of oncology.
Despite the promising insights from this research, experts caution that the development of effective drugs remains a complex challenge. William Nelson from the Sidney Kimmel Comprehensive Cancer Center remarked on the ongoing struggle to transition from basic discoveries to actual drug applications, emphasizing the potential collaborative power of artificial intelligence in this journey. Achieving the capability to rapidly devise tailored treatments based on individual cancer profiles could revolutionize patient care, but significant work still lies ahead.
As the research community continues to harness avenues like neutron experiments for deeper insights into biochemical processes, the hope of transforming cancer treatment and improving patient outcomes becomes more tangible. The results regarding SHMT mark a significant step forward in understanding how to effectively intercept the metabolic hijacking that occurs in cancer cells, paving the way for a future where cancer therapies are not just reactive, but strategically preemptive.

Leave a Reply