In recent years, the increasing levels of carbon dioxide (CO2) in our atmosphere have undeniably sparked concern regarding their effects on global warming and climate change. However, emerging research sheds light on another critical aspect: the influence of CO2 on human cellular functions. This article delves into how elevated CO2 interacts with oxidative stress markers within our cells, specifically through the formation of a potent oxidant known as peroxymonocarbonate, unveiling both its implications for biology and the tools developed to study it.
CO2, a byproduct of cellular respiration, is present both in the atmosphere and within our bodies, serving various roles in biological processes. Recent investigations by Ohara Augusto, a prominent professor at the University of São Paulo’s Chemistry Institute, have unveiled that elevated atmospheric CO2 does not only contribute to climate change but may also generate significant physiological consequences. Studies indicate that high levels of CO2 engage with hydrogen peroxide (H2O2)—a molecule crucial for cellular signaling and oxidative stress responses—forming peroxymonocarbonate, which can drastically influence cellular behavior.
The relationship between CO2 and oxidative stress arises from the gas’s capacity to modify the activity of vital reactive oxygen species. While hydrogen peroxide naturally mediates several cellular functions, its conversion in the presence of excessive CO2 leads to potentially harmful byproducts. Interestingly, research suggests that peroxymonocarbonate can exist within our cells under physiological conditions, adding complexity to the redox chemistry involved in cellular signaling pathways. Understanding this interaction is vital, as it opens new dialogues on how our environment can disrupt cellular homeostasis.
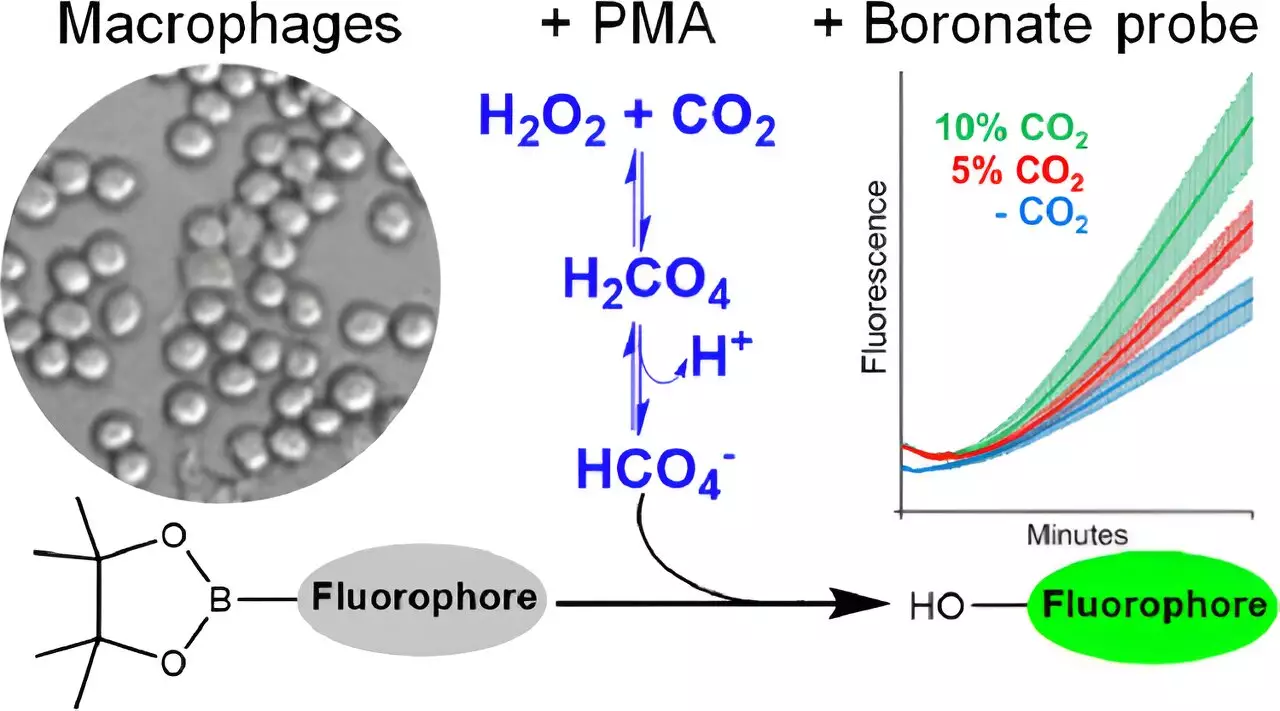
Notably, Augusto and his research group have pioneered a groundbreaking approach to detect peroxymonocarbonate within cells using fluorescent molecular probes. This novel detection method not only highlights the existence of this oxidant in biological systems, previously deemed implausible due to its low formation rates, but also emphasizes the necessity of measuring its physiological relevance. Utilizing boronate-based fluorescence probes, the research team proved effective in identifying the interplay of hydrogen peroxide and CO2 under controlled laboratory conditions.
The experimental design took an insightful approach by activating macrophages—immune cells known for generating reactive species. Through rigorous testing, the researchers concluded that, contrary to expectations, while peroxynitrite and hypochlorous acid were not produced, peroxymonocarbonate indeed emerged as a significant product of CO2’s influence on cellular reactive oxygen species. This discovery plays a crucial role in re-evaluating existing knowledge surrounding redox signaling and the potential os collateral effects posed by incrementally rising atmospheric CO2 levels.
What makes peroxymonocarbonate particularly compelling is its dual potential in redox signaling and cellular dysfunction. Under typical physiological conditions, cells experience mild oxidative stress that helps to sustain adaptability. For instance, the formation of oxidative species like hydrogen peroxide can induce the expression of protective genes, enhancing the production of antioxidant enzymes to combat elevated stress levels. However, this ‘adaptive’ response can become detrimental if excessive amounts of oxidants like peroxymonocarbonate are produced, leading to irreversible cellular damage.
The intricate balance between the beneficial and harmful effects of oxidative states becomes crucial. While some pathways promote cellular resilience, the disruption of this balance due to environmental factors like increased CO2 can incite a cascade of pathological conditions. Thus, understanding how elevated CO2 modulates redox activities could provide insight into a broader array of diseases and health outcomes that are perhaps exacerbated by environmental changes.
The broader implications of Augusto’s research extend beyond immediate scientific intrigue; they provoke fundamental questions about public health and environmental policies. As urban environments continue to grapple with increasing CO2 levels, the physiological repercussions on human health warrant keen attention. The identified role of peroxymonocarbonate signifies a sentinel for detecting the biological effects of heightened CO2 concentrations, ultimately prompting further studies into its varied impacts.
Moreover, as researchers strive to unravel the complexities of cellular redox systems, the necessity for additional empirical evidence regarding peroxymonocarbonate’s roles and mechanisms cannot be overstated. Future research directions should also investigate the molecular pathways that govern the oxidative stress response in the presence of both CO2 and peroxymonocarbonate. Addressing these unexplored interactions will be essential not only for advancing cellular biology but also for developing interventions that mitigate the detrimental effects of increased CO2 exposure on human health.
Understanding the connection between environmental CO2 levels and cellular function is imperative. As research progresses, the recognition of peroxymonocarbonate reveals both a novel area of scientific inquiry and a critical nexus addressing the intersection of environmental science and human health.

Leave a Reply