Recent advancements in our comprehension of cholesterol’s role in cell membranes have emerged from a noteworthy study at Rice University, orchestrated by researcher Jason Hafner. This investigation sheds light on cholesterol’s impact on the structural dynamics of cell membranes, an area critical for understanding diseases such as cancer, where membrane organization is pivotal. By dissecting how cholesterol integrates into biomembranes, this study lays the groundwork for future inquiries into various health implications linked to membrane anomalies.
Cholesterol, a vital lipid molecule, is an integral component of biomembranes which are primarily formed from proteins and lipids. It serves not only as a stabilizing agent but also as a regulator of various cellular processes by influencing the behavior of embedded receptors. This dual functionality underscores the need to unravel the molecular intricacies of cholesterol within its natural lipid environment. Historically, grasping these complexities has eluded researchers, as the interactions between cholesterol and biomembrane components are intricate, necessitating advanced analytical techniques for deeper exploration.
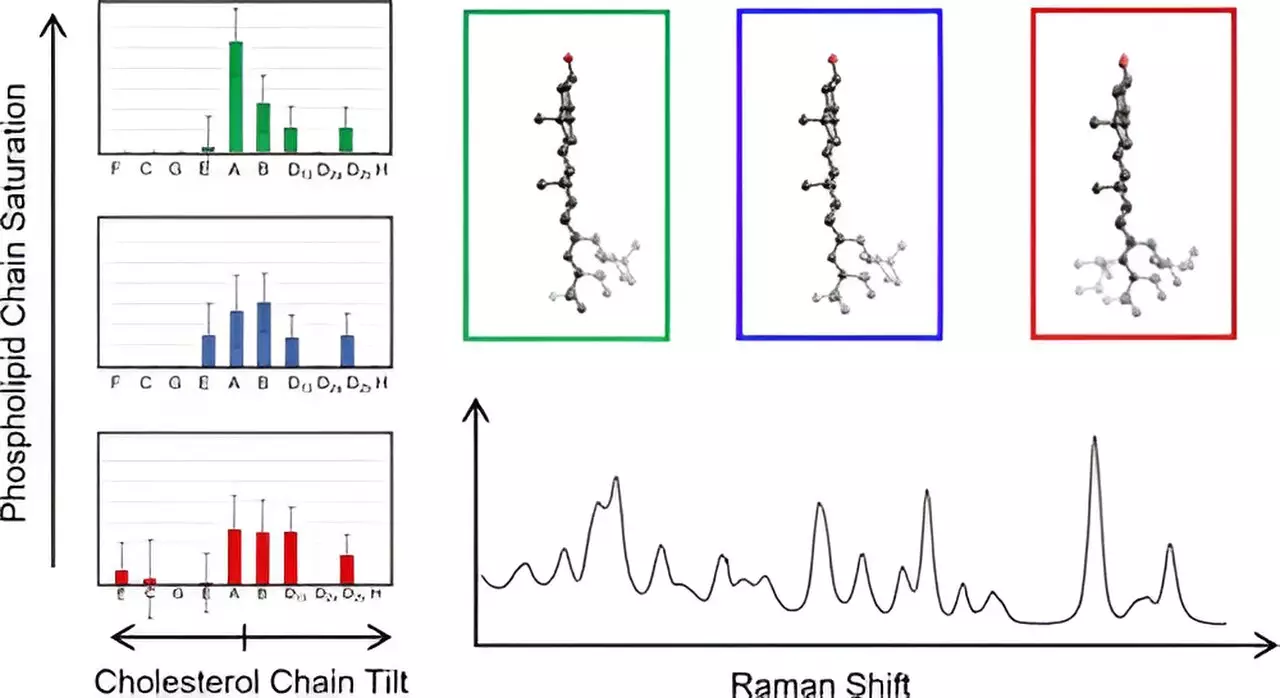
Hafner’s research team harnessed the power of Raman spectroscopy—a revolutionary technique that leverages laser light to elicit vibrational information from molecules. This method affords a meticulous analysis of cholesterol molecules residing within membrane structures. By juxtaposing experimental spectra with those deduced from density functional theory, a quantum mechanical computational method, the researchers gained unprecedented access to the unique spectral signatures of cholesterol amidst the complex lipid landscape of membranes.
The extensive analysis encompassed 60 distinct cholesterol structures, with particular emphasis on its novel fused ring composition and the associated eight-carbon chain. The outcomes of this meticulous study evidenced an unexpected structural categorization among cholesterol molecules, predicated on variations in how the carbon chain diverged from the planar arrangement of the fused rings. This discovery not only expands our understanding of cholesterol’s functionality but also reveals previously uncharted variations in its structural configuration.
The findings are substantial—the research represents the first empirical observation of cholesterol’s chain structures within the membrane milieu, raising the possibility of new avenues for exploring membrane-related diseases. Hafner expressed astonishment over the uniform spectral characteristics observed across different cholesterol molecules within the same structural group, an observation that facilitated a streamlining of analysis. This discovery offers a significant leap in membrane biophysics, fostering a better understanding of how cholesterol’s arrangement can influence cellular functions and potentially lead to dysfunctions often seen in pathologies like cancer.
The diverse research team, which included physics graduate student Kyra Birkenfeld and bioengineering undergraduate student Tia Gandhi, exemplifies a collaborative effort towards advancing biochemical knowledge. With such findings, the results of Hafner’s study are poised to embolden further research aimed at delineating the critical intersection between membrane architecture and disease manifestation, ultimately enriching our approach to medical challenges linked to cell membrane dynamics.

Leave a Reply