Imagine you’re at home, staring at an instruction-free assembly of parts from IKEA—separate pieces yearning to become a functional entity. What if the secrets to assembling these items could be unveiled by understanding how viruses and biological structures naturally combine? This principle exemplifies the burgeoning field of supramolecular chemistry, which studies how smaller molecular building blocks can self-assemble into larger, complex structures. This unique ability is crucial not only in the context of assembling furniture but also for constructing proteins, cellular membranes, and even whole viruses.
Supramolecular chemistry paves the way for advancements in creating “smart materials,” which can respond and adapt to environmental changes—like temperature or chemical presence. Researchers are continuously exploring how adjusting attraction strengths among polymers can produce these tiny masterpieces at the molecular level. Yet, despite its potential, many facets of supramolecular chemistry remain enigmatic. Recent research from Osaka University has illuminated some of these complexities, showing how specific additives can enhance the self-assembly of spherical microparticles made from poly(sodium acrylate), a super absorbent polymer.
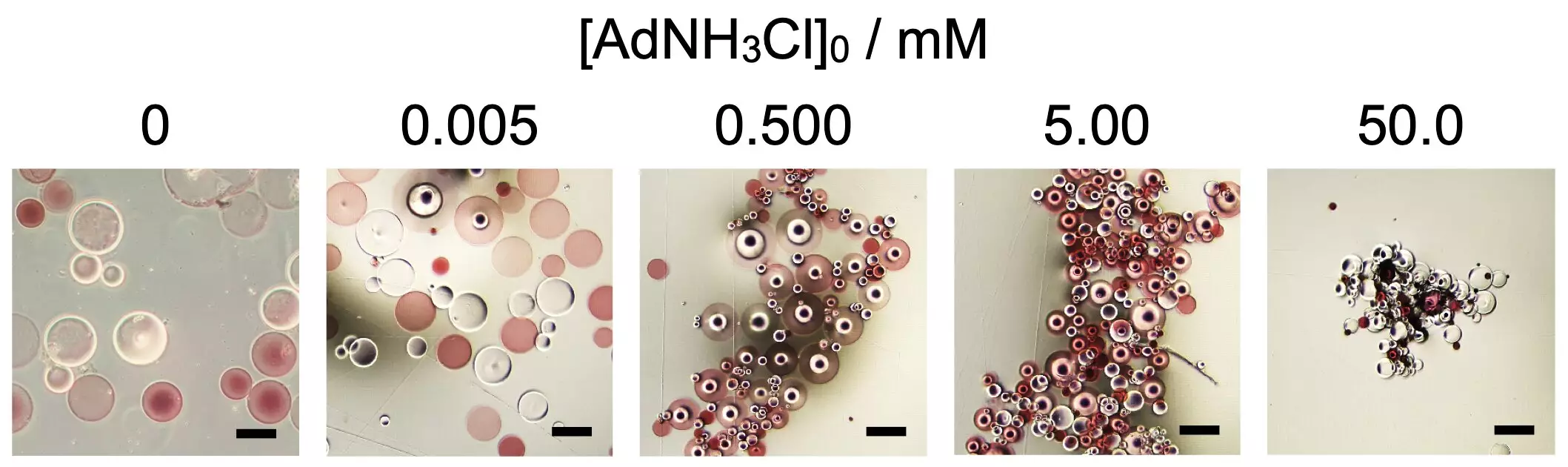
In their groundbreaking study, researchers focused on the impact of chemical additives, particularly 1-adamantanamine hydrochloride (AdNH3Cl), on the assembly process. The polymer microparticles were functionalized using beta-cyclodextrin (βCD) and adamantane (Ad) residues, catalyzing their collective organization. The key finding was that without introducing a critical concentration of AdNH3Cl, these microparticles remained unassembled. This insight underscores the importance of not just the materials themselves, but also the environmental context in which they exist, echoing principles seen in biological systems.
Biological proteins, built from amino acids through a process of folding, can reveal a lot about supramolecular interactions. Just as certain attractions—like hydrogen bonds or hydrophobic interactions—define the shape and function of proteins, the findings suggest similar principles can apply to synthetic polymers. As biological organisms are essentially collections of sophisticated supramolecular polymers, understanding how these polymers assemble offers immense potential for both biological and technological innovations.
The research from Osaka University does not merely contribute to the academic landscape but opens doors to practical applications. Understanding how macroscopic assemblies can be fine-tuned based on microscopic interactions paves the way for developing innovative materials that can respond to external stimuli. As senior author Akira Harada articulates, such insights may help us comprehend the myriad of forms found in living organisms, potentially revolutionizing how we approach material science.
This exploration into the assembly of materials—whether they be in living beings or synthetic constructs—profoundly reshapes our understanding of complexity factors in both nature and technology. By bridging these fields, researchers are setting the stage for remarkable advancements that will enhance the functionality and adaptability of materials in a myriad of applications, from healthcare to engineering.

Leave a Reply