In a noteworthy advancement for the field of biomedicine, researchers from the University of Warwick and the University of Manchester have unveiled a novel computational framework that enhances the freezing of medicines and vaccines. This development shines a light on a crucial aspect of medical science: the preservation of temperature-sensitive treatments such as vaccines, fertility materials, blood products, and oncological therapies. Traditionally, the freezing process, essential for maintaining the efficacy of these treatments, poses significant challenges due to the risk of ice crystal formation. Fortunately, the introduction of a new method grounded in machine learning offers promising solutions and may redefine the future of cryopreservation.
At the core of effective cryopreservation lies a group of substances known as cryoprotectants. These molecules serve the vital function of safeguarding cellular structures during the freezing and thawing processes. Without them, the lifespan of critical treatments is drastically reduced, limiting their use in emergencies. The utilization of cryoprotectants is not just a protocol but a life-saving measure that ensures the sustainability of treatments until they can be administered. However, identifying and optimizing these molecules, which prevent ice formation while maintaining biocompatibility, has usually involved lengthy and costly experimentation.
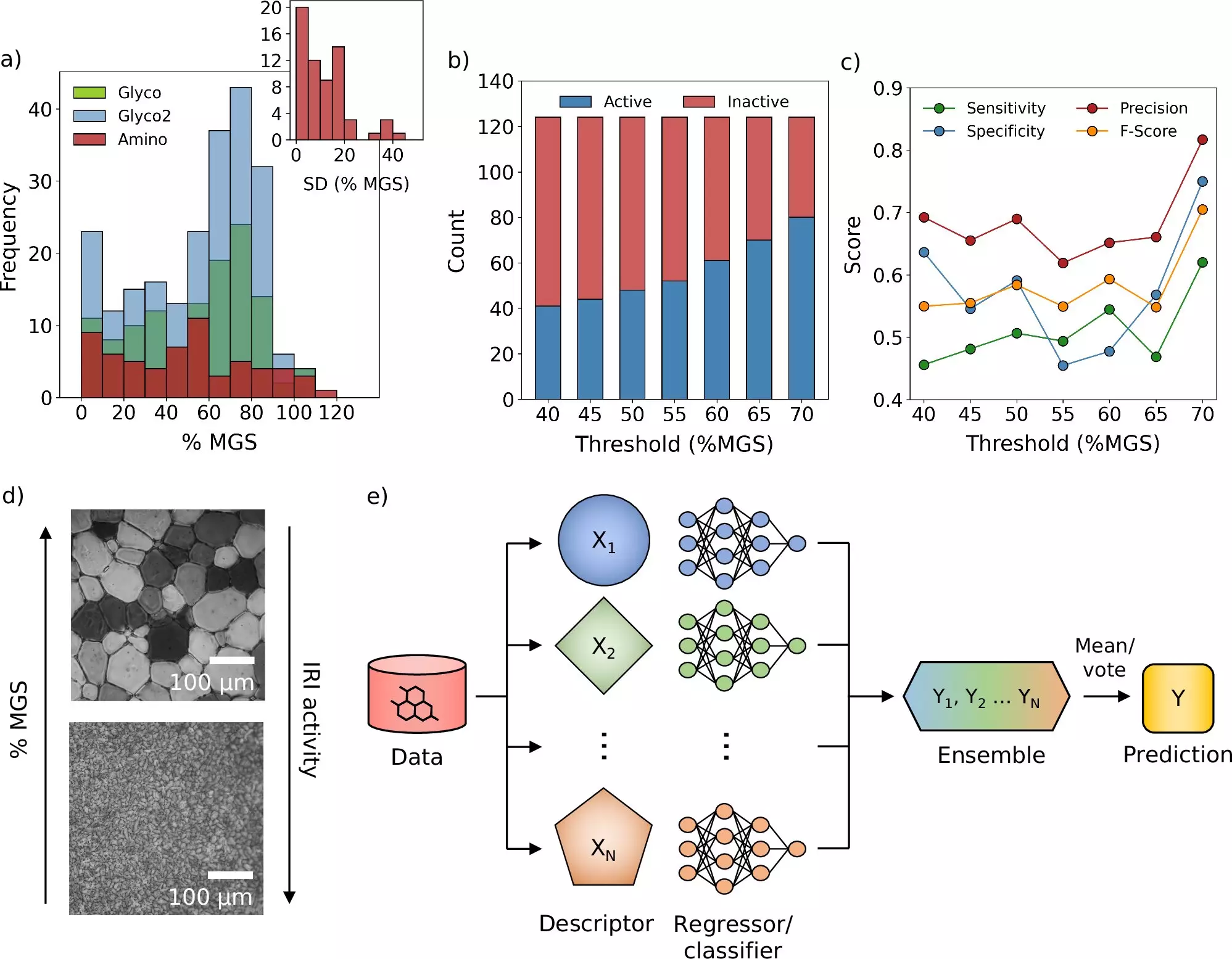
Published in the esteemed journal Nature Communications, the researchers’ new framework employs a machine learning-based model that allows for the virtual assessment of numerous potential cryoprotectants. According to Prof. Gabriele Sosso, who spearheaded the research, this computational strategy should not be perceived as a standalone solution but rather as an adjunct to established methods like molecular simulations and empirical studies. The synergy between these approaches served not only to expedite the process of discovery but also to yield a more precise evaluation of candidate molecules, effectively transforming the methodology of cryoprotectant research.
Dr. Matt Warren, the Ph.D. student who led the day-to-day work, emphasizes that the new model allows researchers to predict the effectiveness of various compounds rapidly. This is a significant leap away from the trial-and-error methods that have historically defined the field. By enabling vast libraries of chemical compounds to be analyzed swiftly, the researchers can prioritize their experimental efforts, concentrating on the most promising candidates—a shift that promises to save both time and resources.
Intriguingly, this research does not merely introduce new molecules but also provides a framework for repurposing existing ones. The findings suggest that molecules previously known may be adapted to serve as cryoprotectants, enhancing their utility and broadening their application in various medical contexts. During the research, the team identified a novel molecule that could inhibit ice crystal growth—an essential goal in the realm of cryopreservation that has remained elusive until now.
Additionally, initial experiments conducted with blood samples unveiled that the quantity of conventional cryoprotectants employed in blood storage could be reduced by incorporating these newly discovered molecules. This could lead to expedited processes in blood transfusion and storage, ultimately benefiting patients requiring swift medical interventions.
The collaboration between teams from the two universities has been described as transformative. Prof. Matthew Gibson, a leading researcher in ice-binding proteins, emphasizes the evolution of their research strategy through partnership with Prof. Sosso. Gibson’s team has spent years uncovering how proteins from polar organisms interact with ice, but the computational modeling has radically streamlined their exploration of new materials and compounds.
This partnership has not only deepened scientific understanding but has also demonstrated the power of interdisciplinary approaches in solving complex medical challenges. As the research continues to unfold, it promises to broaden the horizon for future innovations in the fields of cryobiology and therapeutic applications. The implications of this work could extend beyond medicine, influencing practices in agricultural biotechnology and food preservation as well.
The groundbreaking research emerging from the University of Warwick and the University of Manchester signifies a pivotal moment in the cryopreservation landscape. By integrating machine learning with experimental data, the scientific community stands poised to reshape how we approach the storage of temperature-sensitive medical treatments. As researchers continue to explore the capabilities of this new framework, the potential for accelerated discoveries in cryoprotectants could vastly improve the effectiveness and accessibility of essential therapies, ultimately saving lives and enhancing healthcare worldwide.

Leave a Reply