The impact of carbon dioxide (CO2) emissions on global warming is an issue of increasing urgency in today’s world. As we seek solutions to mitigate climate change, the focus has turned to innovative methods for CO2 capture and sequestration. Cement-based materials, which are integral to construction and infrastructure, have emerged as promising candidates for carbonation processes—transforming CO2 into stable mineral forms. This article delves into the mechanism of carbonation in cement-based materials, recent research advancements, and the implications of such findings in the quest for sustainable building materials.
Understanding Carbonation in Cement-Based Materials
Carbonation is the process through which CO2 interacts with mineral compounds found in cement pastes. When CO2 is dissolved in water, it engages with calcium silicate hydrates (C-S-H), a fundamental component formed during the hydration of cement. This chemical interaction leads to the creation of carbonate ions (CO3^2-) that subsequently react with calcium ions (Ca2+) to form calcium carbonate precipitates.
Despite the promise that carbonation holds for reducing atmospheric CO2, the rigorous nature of the reactions and the composition of cement pastes present complex challenges. One key obstacle is that the carbonating efficiency is influenced by numerous factors—such as relative humidity (RH), the solubility of CO2, and the calcium-to-silicon (Ca/Si) ratio in C-S-H—the basis of a thorough understanding of carbonation mechanisms that remains elusive.
Recent studies, particularly that by Associate Professor Takahiro Ohkubo and his research team from various prestigious universities in Japan, shed light on the intricacies of the carbonation mechanism. Their research, published in *The Journal of Physical Chemistry C*, employed cutting-edge techniques like 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry to enhance our understanding of water transport and structural changes during carbonation.
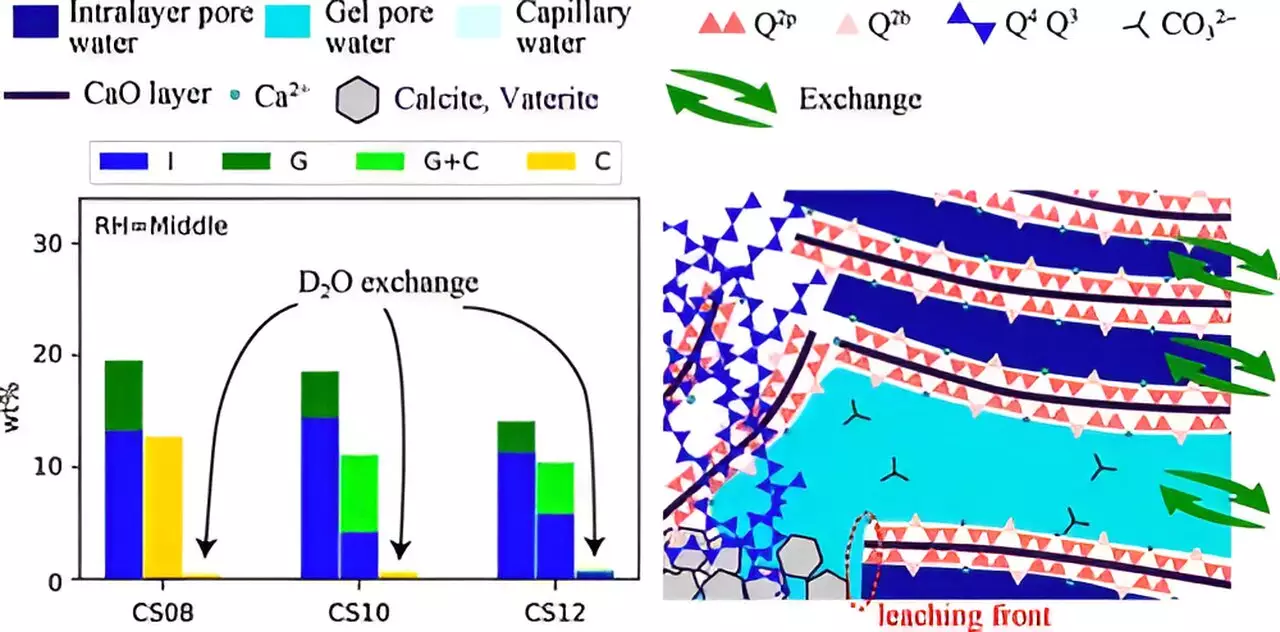
One of the highlighted aspects of their research is the use of accelerated carbonation testing, which allows for initial investigation under conditions that replicate a higher concentration of CO2 than is typically found in the atmosphere. By synthesizing C-S-H compounds under varying RH and Ca/Si ratios, they were able to simulate long-term carbonation processes within a laboratory setting.
Structure, Water Transport, and Carbonation Efficiency
The team’s findings revealed that carbonation leads to notable structural transformations in C-S-H, notably the breakdown of chain structures and alterations in pore sizes. These changes are contingent upon the Ca/Si ratio and RH levels. Interestingly, lower RH and a higher Ca/Si ratio produced smaller pores, which inhibited the movement of Ca2+ ions and water from the interlayer space to the gel-pores. Consequently, this restricted movement can lead to suboptimal carbonation efficiency.
This perspective resonates with a key takeaway from the study: carbonation is not merely a consequence of structural changes but is significantly influenced by mass transfer dynamics. It becomes evident that the interplay between these two factors must be understood in tandem to formulate better carbon-capturing materials.
The implications of Associate Professor Ohkubo’s findings are multi-faceted. As the construction industry constantly seeks to minimize its carbon footprint, these insights can pave the way toward developing innovative building materials capable of ingesting considerable quantities of atmospheric CO2. This push for sustainable practices, driven by research into carbonation mechanisms, places cement-based materials on a trajectory toward future resilience against climate change.
Moreover, the research could also extend beyond the confines of construction. Carbonation is a common phenomenon that occurs within natural organic matter, thereby contributing to our understanding of environmental interactions with CO2. Applying these findings can enhance biogeochemical models that evaluate carbon cycles in various ecosystems.
As evidenced by recent advancements in the understanding of carbonation in cement-based materials, significant progress has been made in tackling the intricate challenges posed by CO2 emissions. The collaborative research effort undertaken by academics from leading institutions reveals the considerable potential of cement-based materials to mitigate climate change through effective CO2 storage.
The study not only elucidates the mechanisms involved in carbonation but also emphasizes the importance of developing materials that effectively harness this process for sustainable practices. As we navigate the challenges of a warming world, the innovations made in understanding and optimizing carbonation could play a pivotal role in our collective effort to reduce atmospheric carbon levels and foster a greener future.

Leave a Reply