The alarming rise of water pollution, particularly due to heavy metals, presents a grave threat not just to human health but also to ecosystems. As industrialization and urbanization escalate, numerous water bodies are overwhelmed with contaminants, leading to dire consequences. Substances like cadmium and lead, when present in drinking water, can result in severe health problems, including neurological damage and organ failure. The traditional methods of filtering these toxins are often inefficient, labor-intensive, and energy-consuming, necessitating the urgent exploration of novel and sustainable solutions.
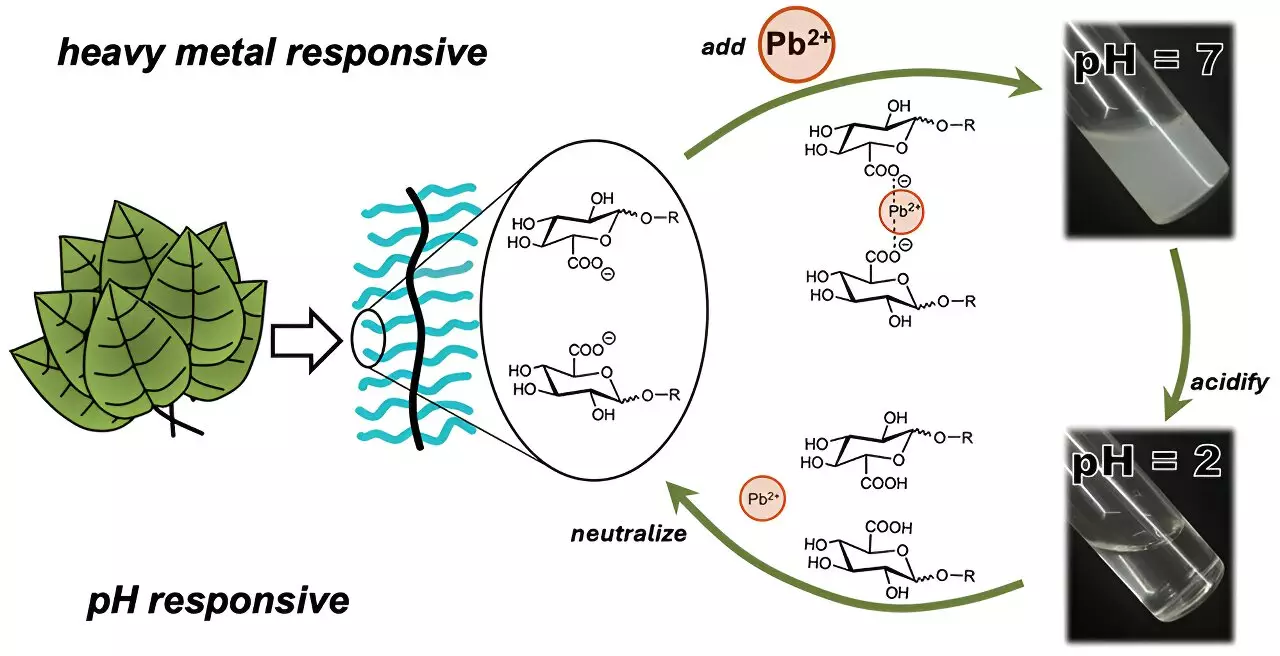
Research indicates that nature, specifically the plant kingdom, holds considerable promise for addressing the heavy metal crisis. Plants utilize intricate polysaccharide structures as the first line of defense against environmental adversities. These structures function by trapping metal ions within their compounds. However, many polysaccharides dissolve in water, which complicates their use in practical applications because additional compounds are often required to immobilize these toxins effectively. Recent advancements have led scientists, like Cassandra Callmann from the University of Texas at Austin, to develop innovative polymers that mimic these natural processes while overcoming traditional limitations.
Callmann’s team has engineered a unique polymer system that combines a water-insoluble backbone with adjustable, sugar-like appendages. The ingenious design resembles a charm bracelet, where each “charm” serves a distinct function in binding heavy metals. The polymer’s ability to selectively trap heavy metals was particularly evident in tests involving ionic cadmium. By incorporating a carboxylic acid group into the polymer structure, researchers found a potent method to attract and bind cadmium ions swiftly, forming clumps that can be easily filtered out.
In rigorous testing, the polymer demonstrated its effectiveness in just three minutes, showcasing significant efficiency in capturing contaminants. What sets this polymer apart is its remarkable ability to release trapped metals by simply adjusting the water’s acidity. After several cycles of capturing, filtering, and releasing, the polymer maintained consistent performance, which is a crucial trait for any sustainable water purification technology.
To further validate the effectiveness of this innovative polymer, the research team shifted its focus toward real-world environments. In a controlled experiment using Colorado River water spiked with ionic cadmium and lead, the polymer displayed its remarkable selectivity and efficiency. Out of the added contaminants, the polymer successfully captured a substantial 20% of cadmium and 45% of lead while filtering out the majority of benign ions such as calcium, sodium, and magnesium. These results underscore the potential for this polymer not just as a theoretical solution but as a practical tool for water purification efforts.
The implications of this research extend far beyond just the removal of heavy metals from water. With global water scarcity becoming an ever-increasing concern, the emergence of efficient and reusable purification methods is crucial. By utilizing renewable materials derived from sugar-like polymers, this technology aligns with sustainable practices and offers an environmentally friendly alternative to conventional purification methods, minimizing both operational costs and energy requirements.
The innovative approach taken by Callmann and her team represents a significant step forward in addressing heavy metal contamination in water sources. Their work illustrates not only the power of nature-inspired solutions but also the importance of interdisciplinary research in tackling some of the most pressing environmental challenges of our time. As further research and development proceed, it is hopeful that these promising materials will pave the way for cleaner, safer water across the globe.

Leave a Reply