As the world grapples with an escalating energy crisis and the pressing need for sustainable solutions, hydrogen energy emerges as a pivotal player. With its clean, low-carbon, and high-energy characteristics, hydrogen presents a compelling alternative to fossil fuels, offering not only energy security but also a significant reduction in greenhouse gas emissions. Among the various methods for hydrogen production, water splitting has gained traction due to its efficiency and potential for large-scale application. Nonetheless, challenges remain, particularly regarding the oxygen evolution reaction (OER), which is fundamental to the electrochemical processes involved in water electrolysis.
Challenges in Oxygen Evolution Reaction
At the heart of the struggle for achieving high efficiency in the OER lies the insufficient kinetics at the anode. This bottleneck impedes the overall energy conversion efficiency, making it imperative to develop effective catalysts that can facilitate this process. The ongoing quest for innovative materials to overcome the sluggish OER rates has led researchers to explore a variety of catalysts, with single-atom catalysts (SACs) gaining prominence due to their unique properties. These catalysts are engineered to have isolated metal atoms dispersed across a support matrix, thus enhancing their interaction with reaction intermediates and improving catalytic performance.
A groundbreaking study conducted by a research team led by Professor Bao Jun at the University of Science and Technology of China has shed light on the potential of cobalt-based oxide-supported, high-density iridium (Ir) single-atom catalysts. Their research, published in the esteemed journal Angewandte Chemie, reveals how these high-density SACs exhibit remarkable catalytic performance when subjected to OER conditions. This advancement signifies a key step forward in the development of more efficient catalysts that could streamline hydrogen production.
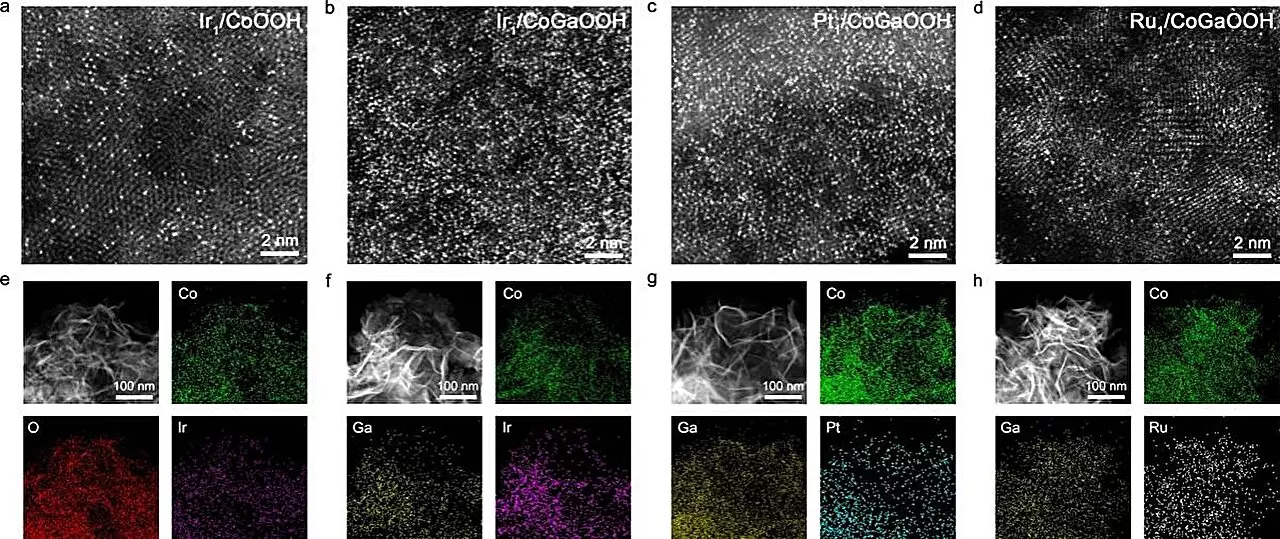
One of the critical findings of this research revolves around the relationship between the density of single atoms on the catalyst’s surface and its overall efficacy. As the density of these metal atoms increases, the proximity between them fosters a phenomenon known as “neighboring synergetic interaction.” This interaction enhances the adsorption behavior of reaction intermediates, leading to superior catalytic performance. The innovative approach taken by Professor Jun’s team involved incorporating gallium (Ga) atoms into the cobalt-based oxide lattice to manipulate the electronic structure of the anchoring sites for the single atoms. This pioneering technique resulted in the formation of high-density SACs, which exhibited significantly improved bonding strength with oxygen defect sites.
The performance of the newly crafted catalysts was rigorously evaluated, showcasing impressive results. The high-density Ir single-atom catalyst, denoted as Nei-Ir1/CoGaOOH, displayed a remarkably low overpotential of 170 mV at a current density of 10 mA cm-2, coupled with exceptional stability exceeding 2000 hours. In further tests, the catalyst demonstrated consistent operation at a current density of 1 A cm-2 in alkaline electrolytes for over 50 hours. In situ Raman spectroscopy analysis indicated that the structural integrity of the catalyst was well-maintained throughout the OER process, highlighting its robustness under operational conditions.
Intriguingly, further investigations into the mechanisms at play unveiled that the dramatic enhancement in catalytic performance was not merely attributable to improvements in the electronic structures of the active sites. Instead, it was the neighboring synergetic interactions among the high-density Ir single atoms that played a crucial role. These interactions stabilize the *OOH intermediates by generating additional hydrogen bonding, which effectively lowers the reaction energy barriers and amplifies the catalyst’s overall effectiveness.
Future Implications and Prospects
The findings from this research not only unlock new avenues for the development of advanced electrochemical catalysts but also provide critical insights into the intricate dynamics of SACs. As the world shifts towards more sustainable energy solutions, the pursuit of efficient hydrogen production methods through innovative catalyst design will remain paramount. This study underscores the potential for future breakthroughs in energy technology, setting the stage for a new era of hydrogen energy that can significantly contribute to global decarbonization efforts.

Leave a Reply