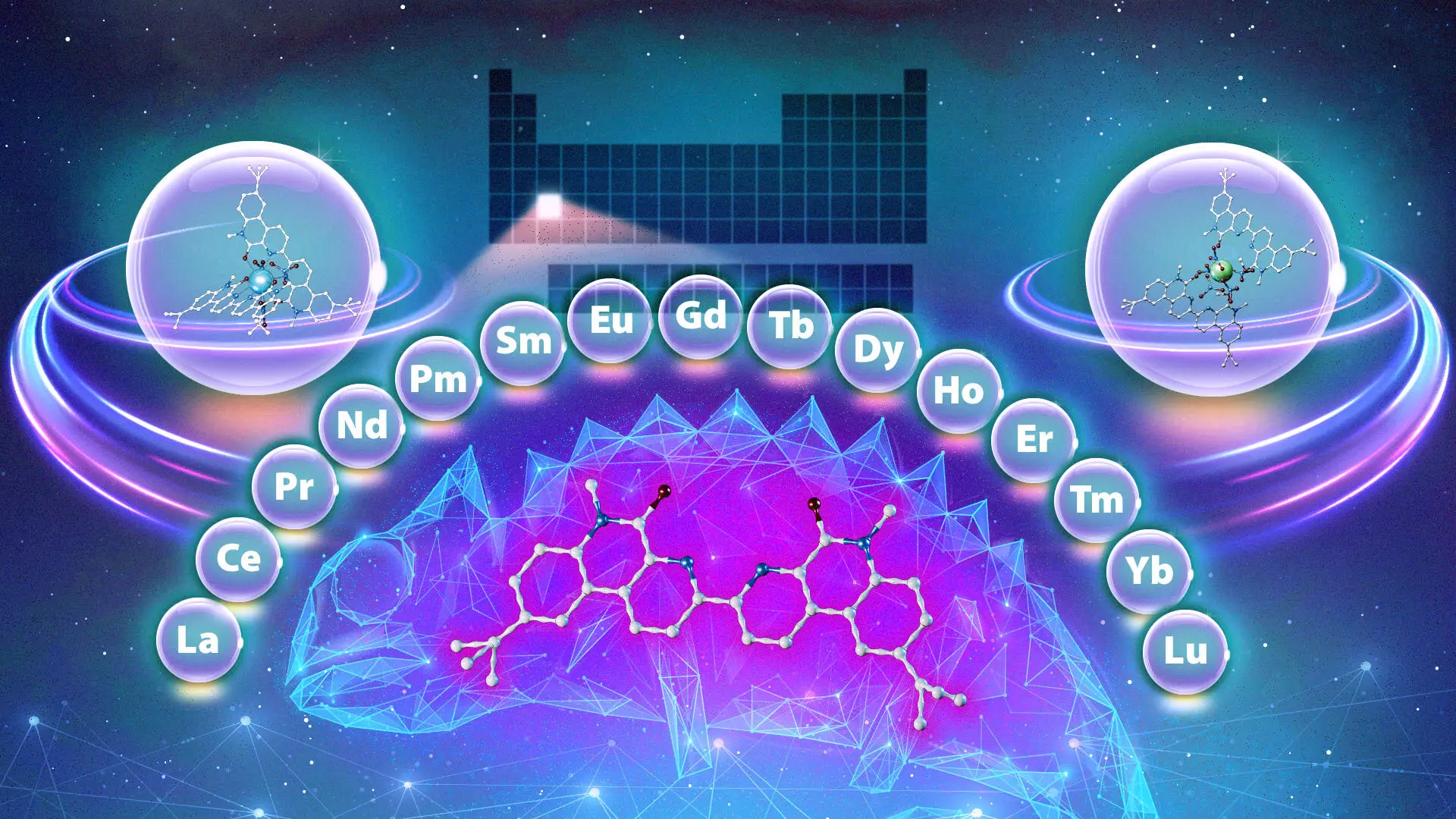
In a groundbreaking study conducted by researchers at the Oak Ridge National Laboratory (ORNL) in collaboration with Vanderbilt University, a novel chemical known as the “chameleon ligand” has been discovered, which has the potential to transform the way rare-earth metals, specifically lanthanides, are purified. Such advancements can significantly impact varied industries reliant on these metals, ranging from renewable energy solutions and healthcare technology to national defense operations. Typically regarded as “rare,” these lanthanides are not exceptionally uncommon in the Earth’s crust; rather, the challenge lies in their complex separation processes which have remained a significant hurdle within the realm of materials science.
The Complexity of Lanthanide Separation
The group of elements known as rare-earth metals consists primarily of 15 lanthanides, along with two other elements. These metals are ubiquitous in nature, comparable in abundance to better-known elements like copper and lead. They possess unique electronic and optical properties that make them essential for numerous applications, from high-strength magnets and phosphors in display screens to catalysts in chemical manufacturing. Nonetheless, extracting pure forms of these metals from their naturally occurring mixtures requires advanced techniques in separation science due to their similar chemical characteristics.
As noted by Subhamay Pramanik, a former ORNL postdoctoral fellow, isolating individual lanthanides is inherently challenging because of their close proximity in size and chemical properties. The purification process typically involves the use of ligands, which are compounds designed to latch onto specific metals in solution. Classic methods of separation can be excruciatingly slow and costly, with a penchant for generating environmental waste—a stark contradiction to the sustainability goals underpinning the burgeoning clean energy sector.
The innovation introduced by the ORNL study revolves around the chameleon ligand, which adapts its behavior based on environmental conditions—much like a chameleon altering its color for camouflage. This ligand exhibits a remarkable ability to selectively bind to different lanthanide ions dependent on factors such as the acidity of the solution and the duration of interaction time. For example, under acidic conditions, the ligand shows a preference for heavier lanthanides, indicating its dual capacity for separation.
Highlighting the potential implications of this discovery, co-lead researcher Santa Jansone-Popova underscores that traditional ligands are generally restricted to preference for either lighter or heavier lanthanides. This unique behavior implies that the chameleon ligand can facilitate multiple separations using a single compound, drastically simplifying the traditional multi-step separation processes. Not only could this streamline the purification workflow, but it also holds promise for reducing the overall environmental footprint by minimizing waste generation.
The chameleon ligand’s versatile capabilities could well revolutionize existing separation processes used in industries that rely on rare-earth metals. By enabling the simultaneous extraction of lanthanides in any order—ranging from heaviest to lightest or conversely—the ligand expedites the overall purification procedure. ORNL’s Ilja Popovs points out that this discovery emphasizes the importance of understanding how slight variations in ligand structure can influence behavior in significant ways, thus broadening the scope of materials Science.
As researchers probe further into the mechanisms of the chameleon ligand, there lies great potential for discovering and developing additional ligands with similar adaptable behaviors. This can lead to breakthroughs in techniques not only for lanthanide purification but also for the extraction of other complex mixtures in the future.
The development of the chameleon ligand marks an important stride in the pursuit of sustainable technologies. As the world increasingly pivots towards clean energy and advanced materials, the efficient and environmentally friendly purification of rare-earth metals becomes crucial. The findings from ORNL represent a convergence of advanced material sciences and sustainability goals, paving the way for new possibilities in metallurgy and resource management. With ongoing investigations into the chameleon ligand’s unique properties, the potential to enhance resource recovery and reduce environmental impacts is greater than ever. This innovation not only pushes the boundaries of knowledge in separation chemistry but symbolizes hope for future advancements in the field of clean technology.

Leave a Reply