Recent advancements in the realm of renewable energy technologies have shed light on the importance of efficient catalysts for the oxygen evolution reaction (OER). Recognized as a key player in processes like water electrolysis and metal-air batteries, catalysts can significantly influence the reaction rates essential for hydrogen production. A dedicated team of researchers has made impressive strides in this area by innovating relatively economical catalysts through the incorporation of chromium (Cr) into transition metal hydroxides. This breakthrough was documented in a study published in the prestigious journal ACS Catalysis on August 30, 2024.
The fundamental challenge with the OER lies in its inherent slow reaction kinetics, often necessitating high-performance and durable catalysts to facilitate the process. Researchers at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) have focused on improving these catalysts by employing chromium doping. Lead researcher Hao Li articulated that integrating chromium facilitates a swift transition of metal hydroxides into a more active state known as oxyhydroxide, which is pivotal for enhancing OER efficiency.
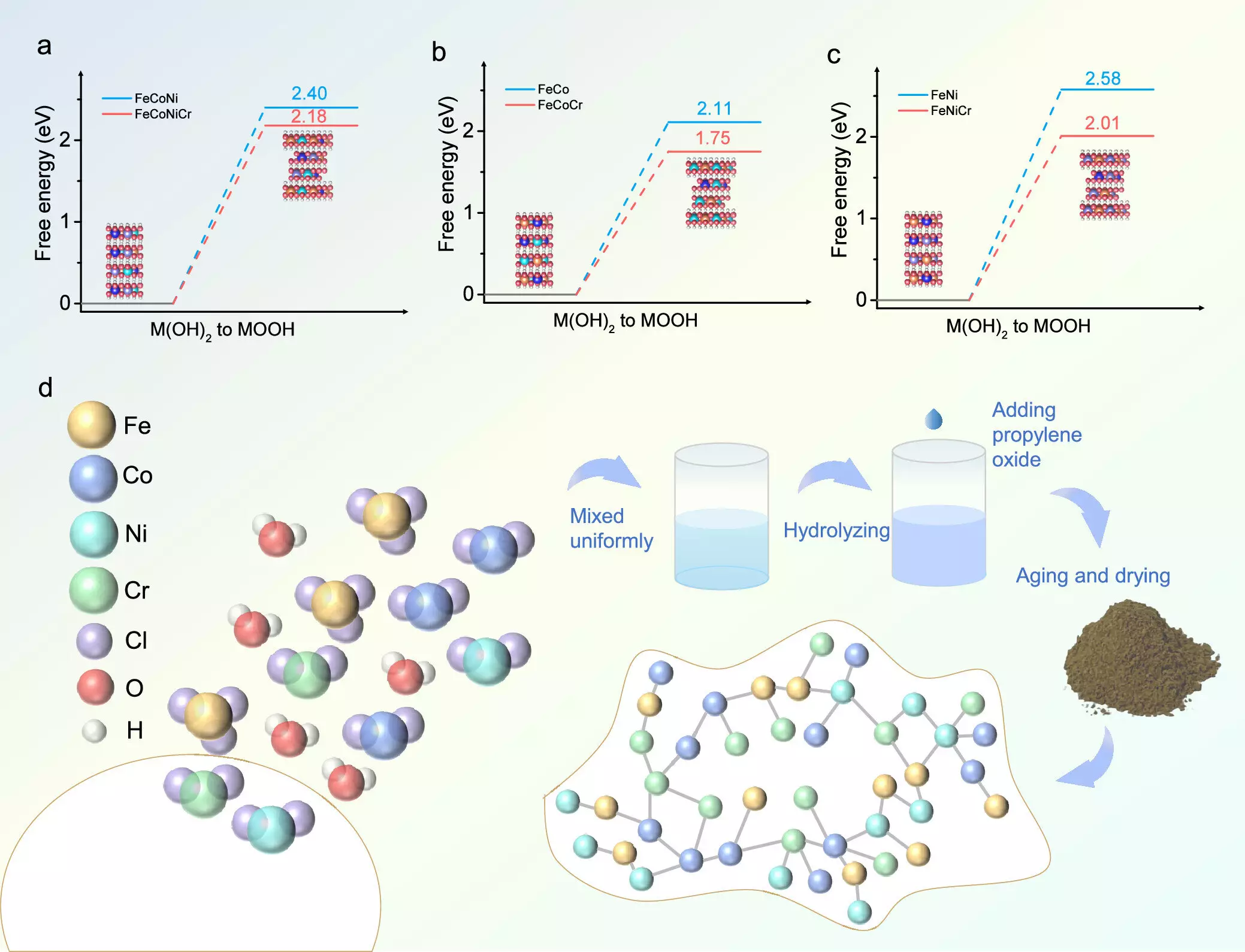
Employing an aqueous sol-gel approach, the research team successfully synthesized a FeCoNiCr hydroxide catalyst that integrates iron (Fe), cobalt (Co), nickel (Ni), and chromium in a uniform structure. The resultant catalyst exhibited a remarkably low overpotential of 224 mV when tested in alkaline environments. This performance surpassed that of similar catalysts by a margin of 52 mV, while also demonstrating impressive stability over an extensive continuous operation period of 150 hours.
Furthermore, when integrated into a zinc-air battery configuration, the FeCoNiCr catalyst maintained stable operation for an impressive duration of 160 hours, with a minimal voltage difference of 0.70 V between discharge and charge cycles. Such sustained performance underscores the catalyst’s potential for practical applications, thus presenting a feasible option for clean energy systems.
The research team’s efforts were bolstered by density functional theory (DFT) analyses, which indicated that doping with chromium optimizes the electronic environment around the catalyst’s active sites. This adjustment enhances the adsorption characteristics, leading to improved efficiency of the OER process. Further analysis revealed that nickel and cobalt sustained favorable oxidation states during the process, contributing to the overall catalytic activity.
As the team looks towards the future, they are setting their sights on investigating additional elements that could further improve the efficacy of these catalysts. Di Zhang, a co-author and assistant professor at WPI-AIMR, emphasized the potential of this research as it not only equips them with a framework for synthesizing enhanced materials but also propels the development of even more effective catalysts capable of facilitating hydrogen production on a broader scale.
The pressing global need for innovative methods of producing clean energy hinges significantly on the advent of efficient and cost-effective catalysts. As researchers continue to unravel the complexities of OER processes and improve catalytic performance, the transition towards sustainable energy solutions appears increasingly attainable. The promising findings from this study play a pivotal role in advancing renewable energy technology, particularly as hydrogen production emerges as a cornerstone for future energy systems.

Leave a Reply