Photocatalysis is a fascinating area of chemistry that mimics natural processes, specifically photosynthesis, to facilitate chemical reactions using light. The core idea behind photocatalysts is their ability to initiate processes which typically require energy-intensive conditions such as extreme temperatures. This capability holds immense potential for both environmental sustainability and industrial applications, particularly in the conversion of renewable resources into valuable chemicals. However, achieving high quantum efficiency—meaning the transformation promoted by the photocatalyst is as effective as possible—is paramount for these systems to be economically viable on a larger scale.
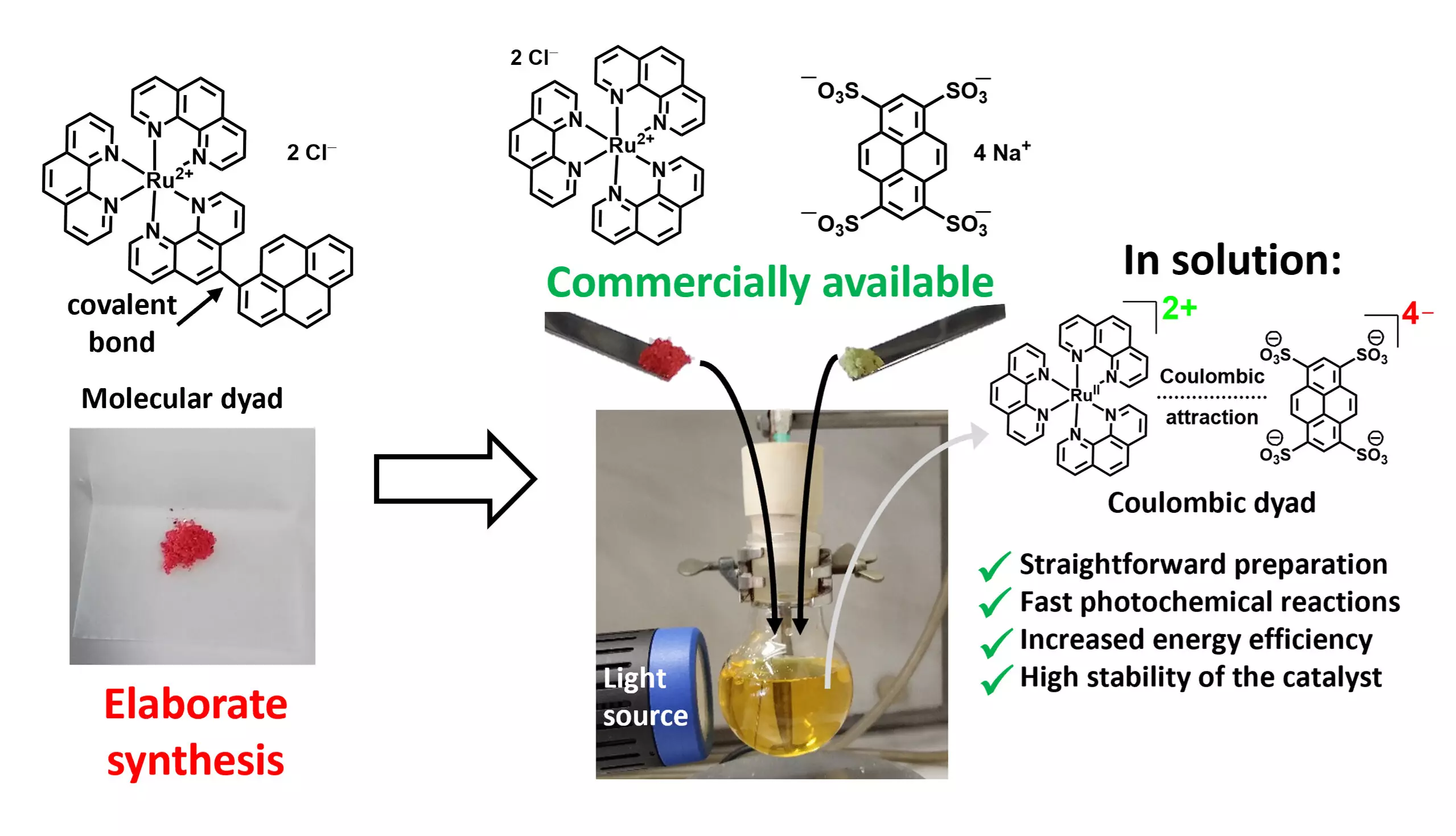
Traditionally, the development of highly efficient photocatalysts has been a complex and costly endeavor, especially when using multi-step syntheses to create advanced molecular dyads. These systems often comprise two photoactive units linked by covalent bonds, which can significantly drive their efficiency but come with high production costs. This limitation discourages their large-scale application, as the expenses and complexities associated with synthesis render them impractical alternatives to traditional catalysts, which frequently rely on precious metals like iridium or osmium.
The quest for alternatives has led to extensive research aimed at transforming non-precious metals into effective photocatalysts. Yet, the challenge remains that even Earth-abundant metals require sophisticated and resource-draining synthesis procedures. This scenario presents a significant barrier to developing economical solutions for photocatalytic processes, particularly in industries aiming to transition towards greener methodologies.
A groundbreaking approach, led by Professor Christoph Kerzig from Johannes Gutenberg University Mainz, has emerged to circumvent these challenges. The team developed a technique that involves mixing two commercially available salts to form ion pairs through electrostatic interactions, or Coulomb interactions. This method stands as a significant simplification from traditional multi-step processes that prompted high costs and complexity.
Essentially, the attractive forces between the ionic components allow for a synergistic interaction among the photoactive units, mirroring the relationships seen in everyday materials such as table salt—where sodium and chloride ions interact. By employing this straightforward methodology, researchers have successfully created a new class of photocatalysts that promises enhanced performance without exorbitant expenses.
Initial testing of these Coulombic dyads has shown promising results, revealing their effectiveness over conventional and more expensive catalysts in reactions such as carbon-carbon bond formation and the photooxygenation of biomass-derived materials. These reactions leverage both sunlight and LED-generated light, demonstrating the potential of this approach to enhance the conversion of light energy into useful chemical products efficiently.
The findings suggest that this methodology is not only effective but also versatile. The researchers believe that the ionic nature of many well-established photocatalysts can be harnessed more effectively using this innovative strategy. By adjusting the choice of solvents, various Coulombic dyads can be synthesized, essentially creating a toolbox approach where different combinations of photoactive components can be explored for optimal performance in specific reactions.
One of the most significant implications of this research is the potential for scaling up photocatalytic reactions for industrial applications. By reducing the amount of catalyst required without compromising efficiency, the Kerzig group’s strategy could yield significant cost savings for industries reliant on photocatalysis. The ability to utilize inexpensive additives to improve the performance of established photocatalysts opens up new avenues for sustainable chemical production at a larger scale.
As industries increasingly shift towards greener and more resource-efficient methods, this innovative approach to photocatalyst design may well pave the way for a future where chemical transformations are not only achievable under mild conditions but are also economically sensible. The promise of creating value-added products sustainably represents a pivotal transition in the realm of chemical manufacturing.
The transformation of traditional photocatalytic systems into more efficient, versatile, and economically viable options heralds a new era in photocatalysis, blending economic practicality with environmental considerations. This evolution could substantially impact how various chemicals are manufactured, aligning with sustainability goals crucial for the future of our planet.

Leave a Reply