Recent advancements in ion chemistry have emerged from a collaborative study conducted by prominent research teams at the Fritz Haber Institute, Sorbonne University, and Uppsala University. Their significant findings, published in *Nature Communications*, shed light on the intricate behavior of ions within solutions, particularly regarding the elusive solvation shells that form around dissolved substances. This newly acquired knowledge is instrumental for various scientific disciplines, laying the groundwork for future research and practical applications.
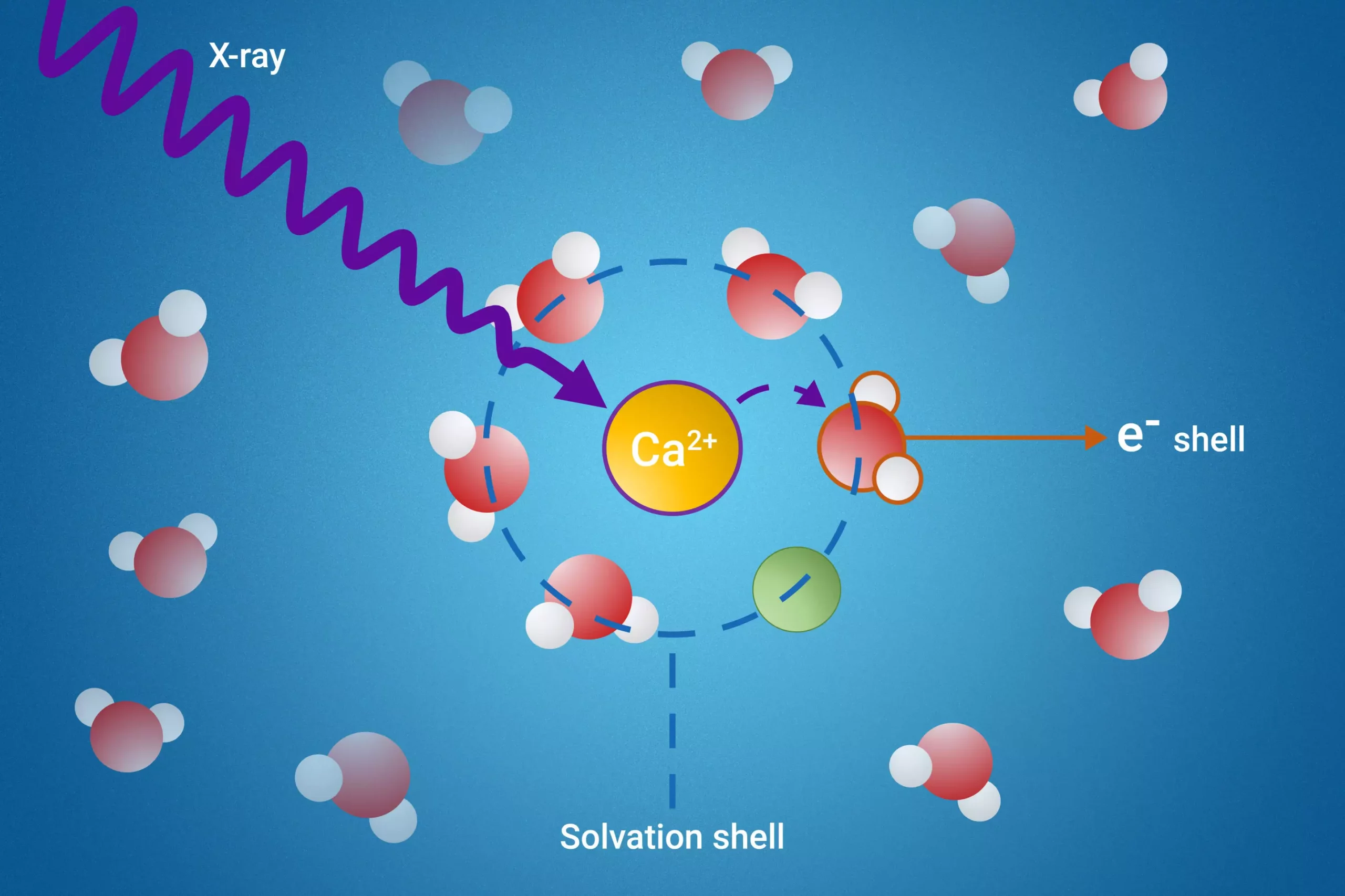
When substances dissolve in liquids, they begin to interact with solvent molecules, resulting in the formation of solvation shells. These shells consist of solvent molecules that become intimately associated with the dissolved particles, effectively altering their properties. Interestingly, molecules in these shells exhibit behaviors and characteristics that differ markedly from those of bulk solvent molecules. Understanding these interactions is critical in fields such as chemistry, biology, and materials science; however, research into solvation shells has been hindered by their complex structures and the challenges of isolating specific solvent molecules from their abundant counterparts.
To tackle this longstanding challenge, the research team has developed a pioneering technique that utilizes resonant intermolecular Coulombic decay (ICD). By employing X-rays to excite the molecules, the scientists can explore how these molecules interact with their neighbors during the decay phase. This refined observation provides unprecedented insight into the unique properties of solvation shells. The ability to examine these interactions marks a significant leap forward in our comprehension of ion behaviors in various environments.
One of the standout findings of this study is the identification of a specific ICD process that serves as a reliable indicator of ion pair formation. Perhaps more notably, the researchers successfully measured the electron binding energies of water molecules located in the first solvation shell. This milestone not only sheds light on previously obscure properties but also enhances our understanding of the stability and dynamics of these critical solvation layers.
The implications of this research extend far beyond fundamental chemistry. By elucidating the nature of solvation shells, this work lays the foundation for novel approaches in material science, atmospheric research, and electrochemistry. Insights into solvation dynamics can aid in the refinement of chemical processes, improve energy storage capabilities, and enhance the design of innovative materials. The new methodology enables researchers to study solvation shells with unprecedented precision, opening doors to further investigations that could revolutionize several scientific and engineering fields.
This groundbreaking study highlights the importance of understanding solvation shells and the profound impact such knowledge has across a multitude of scientific endeavors. The efforts of the research community not only deepen our theoretical understanding but also pave the way for practical applications that could benefit various technological advancements.

Leave a Reply