Dinitrogen (N2), constituting approximately 78% of Earth’s atmosphere, presents an intriguing possibility for innovations in chemical synthesis. Despite its abundance, harnessing dinitrogen in practical applications proves difficult due to the robustness of its triple bond. The high stability of this molecular bond renders direct participation in chemical reactions a formidable challenge. The process traditionally used to convert dinitrogen into useful compounds, notably for manufacturing alkyl amines necessary for pharmaceuticals and polymers, involves the Haber-Bosch process. This long-standing method first splits N2 to produce ammonia, followed by further transformations to activate alkene pre-cursors, ultimately increasing both the duration and energy costs of synthesis.
The current procedures employed in synthesizing nitrogen and carbon derivatives are energy-intensive and time-consuming. Takanori Shima, a leading researcher at the RIKEN Center for Sustainable Resource Science, underscores the pressing need for a more streamlined approach. “Using dinitrogen and alkenes directly to construct alkyl amines under less strenuous conditions would be vastly preferable,” he asserts. Yet, achieving such a synthesis has remained elusive, with expectations of significant difficulties standing in the way of advancements within this field. This persistent gap highlights the necessity for innovative methodologies that can harness readily available resources without compromising efficiency or increasing operational costs.
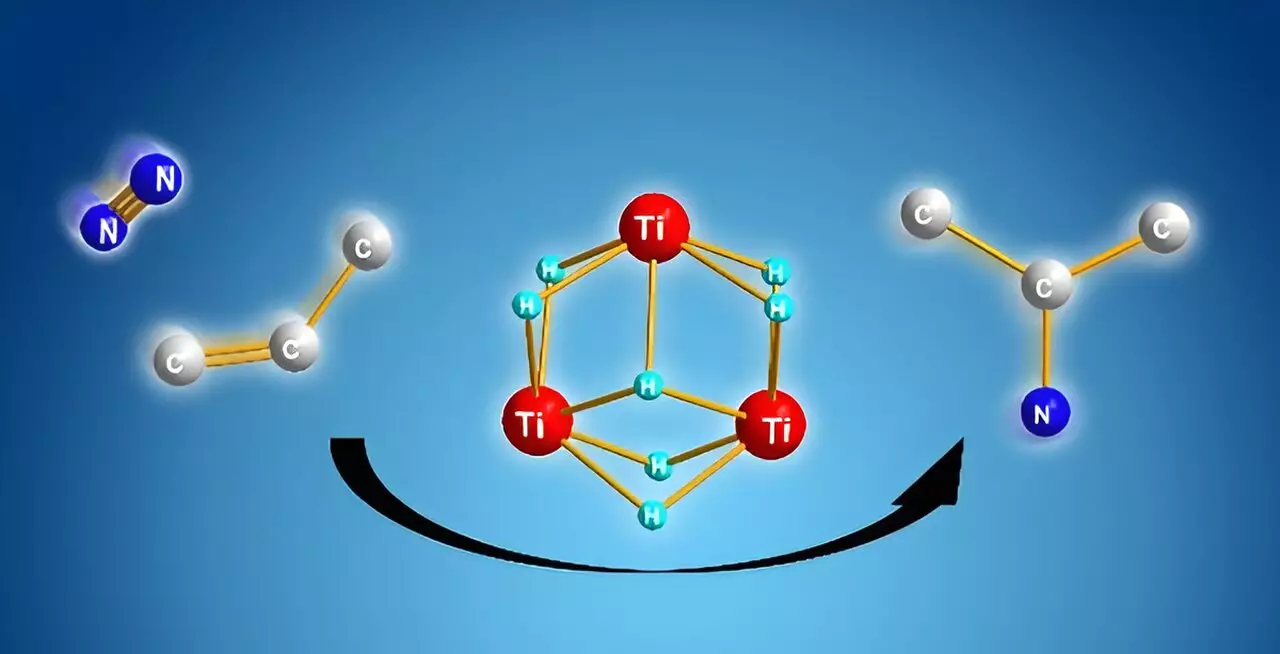
A groundbreaking revelation came when Shima and his team explored the potential of titanium polyhydrides—a novel class of chemical complexes made up of interconnected titanium atoms through hydrogen bridges. Their prior research had indicated that these complexes exhibit heightened reactivity not just toward dinitrogen, but also toward other stable small molecules. What was particularly striking was that these titanium-hydride units could cooperate effectively during chemical reactions, leading to promising avenues for direct synthesis of alkyl amines.
The researchers’ experiments revealed an impressive mechanism: upon reacting alkenes with titanium polyhydride, the alkenes underwent activation, leaving several titanium–hydride units available for subsequent reactions. When dinitrogen was introduced, these reactive units facilitated the cleavage of the nitrogen molecule, leading to the formation of new nitrogen–carbon bonds through a cooperative reaction. This insight not only simplifies the synthesis process but effectively reduces the energy required, addressing the inefficiencies associated with conventional methods.
Computational Insights and Future Directions
Crucially, computational analyses played a role in understanding the underlying mechanisms of the reaction. It was established that once the substrates—the alkenes and dinitrogen—were activated, the formation of nitrogen–carbon bonds occurred preferentially within the titanium polyhydride framework. This is particularly significant as nitrogen–carbon bond formation proves to be more energetically favorable compared to forming other types of chemical bonds, such as nitrogen–hydrogen or carbon–hydrogen bonds. This discovery not only elucidates the reactivity patterns of the system but also positions titanium polyhydrides as a promising platform for innovation in synthetic chemistry.
Shima’s research team is keen to build on these findings, aspiring to transition this groundbreaking reaction into a catalytic process. By developing a catalytic framework based on these principles, the potential exists to create a sustainable and efficient method for transforming abundant resources into high-value compounds, ultimately leading to numerous industrial applications.
Implications for Industrial Chemistry
The implications of harnessing dinitrogen in this new framework are profound. If researchers can establish a catalytic method for the direct synthesis of alkyl amines from dinitrogen and alkenes, the chemical industry may see significant reductions in energy consumption and time expenditure. This could pave the way toward more sustainable production methodologies that lower the carbon footprint of chemical manufacturing processes, fundamentally shifting how we approach resource utilization in industrial settings.
As Takanori Shima and his team continue their important work, the chemical community waits with anticipation for what this innovative pathway could mean for the future of synthetic chemistry, particularly in the realms of pharmaceuticals and material science.

Leave a Reply