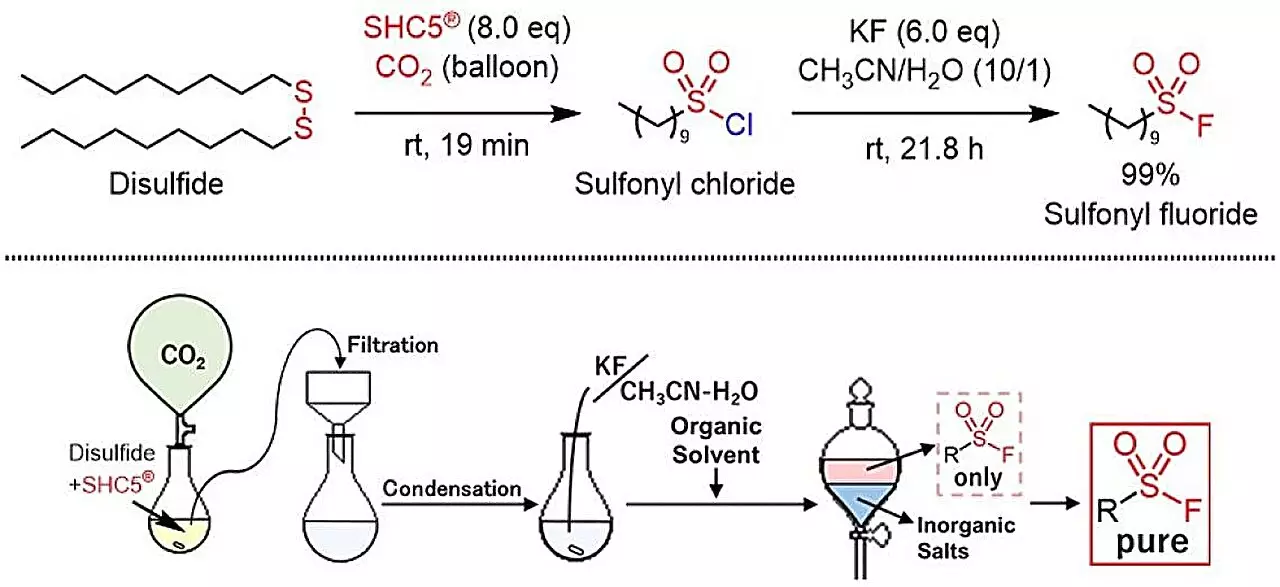
Recent advancements in click chemistry have led to a novel method for synthesizing sulfonyl fluorides from thiols and disulfides, utilizing the benign reagents SHC5 and KF (potassium fluoride). This innovative process marks a significant evolution in the field of organic synthesis, promising not only high efficiency but also a low environmental footprint, as it produces only innocuous byproducts: sodium chloride and potassium chloride. The salient research findings were highlighted in an article published in ACS Sustainable Chemistry & Engineering, showcasing the method’s potential impact on both the chemical industry and pharmaceuticals.
Click chemistry has garnered attention in recent years for its versatility and efficiency in assembling complex molecular architectures. Renowned for its high selectivity and rapid reaction times, click chemistry is increasingly utilized across several fields including material science, chemical biology, and drug development. The appeal of this synthetic approach lies in its ability to facilitate the construction of molecular frameworks with minimal byproducts, thereby adhering closely to the principles of green chemistry. The introduction of sulfonyl fluoride compounds into this domain not only amplifies its utility but also enhances the existing repertoire of click chemistry reactions.
Historically, synthesizing sulfonyl fluorides involved using hazardous agents such as SO2F2 gas or potassium hydrogen fluoride (KHF2), both of which pose significant safety risks and environmental concerns. The reliance on such toxic reagents raises issues surrounding the practicality of widespread application in laboratories and industrial settings. Consequently, chemists have prioritized the search for safer, more sustainable alternatives that can replace these harmful substances and address regulatory and safety challenges in chemical manufacturing.
The new methodology employs SHC5 in conjunction with KF to facilitate the efficient generation of sulfonyl fluorides from readily available thiols and disulfides. This approach stands out due to its simplified procedure, rendering it adaptable for low-cost, scalable production. By eliminating the need for toxic reagents, the protocol not only demonstrates a commitment to environmental stewardship but also positions itself as a pioneering technique within the chemical synthesis landscape. The ability to create a diverse range of sulfonyl fluorides—spanning aromatic, aliphatic, and heterocyclic compounds—further widens the potential applications of this method.
The implications of this research extend beyond the immediate benefits of improved chemical processes. According to the study’s authors, Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem, the drive to innovate sustainable organic synthesis methods correlates with broad objectives such as the Sustainable Development Goals (SDGs). As the scientific community faces an increasing imperative to mitigate environmental impact, developing safer reaction protocols that align with these global goals will be crucial. This study represents a meaningful stride forward in not only advancing chemical synthesis but also in promoting practices that are conscious of ecological consequences.
The transition to eco-friendly methodologies in the synthesis of sulfonyl fluorides exemplifies the necessary evolution within the field of chemistry. By prioritizing both efficiency and environmental safety, this innovative approach not only enhances existing synthetic protocols but sets a compelling precedent for future research and development in sustainable chemistry.

Leave a Reply