Recent research from the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig has sparked a new realm of inquiry into atmospheric chemistry by revealing the presence of sulfurous acid (H2SO3) in the gas phase for the first time under atmospheric conditions. This remarkable study, published in the prestigious journal Angewandte Chemie, challenges long-standing assumptions about the rarity and elusive nature of sulfurous acid, which has primarily been dismissed as difficult or even impossible to isolate.
Textbooks have typically accepted that H2SO3 may exist transiently in aqueous solutions containing sulfur dioxide (SO2), but its existence as an isolated compound has been regarded as improbable. Efforts to experimentally detect H2SO3 in such solutions have repeatedly proven unsuccessful, yielding only its corresponding salts—bisulfite (HSO3–) and sulfite (SO3^2–). The only prior experimental observation of sulfurous acid was recorded by Helmut Schwarz’s team at TU Berlin in 1988, who could only detect it under specific conditions concerning mass spectrometry.
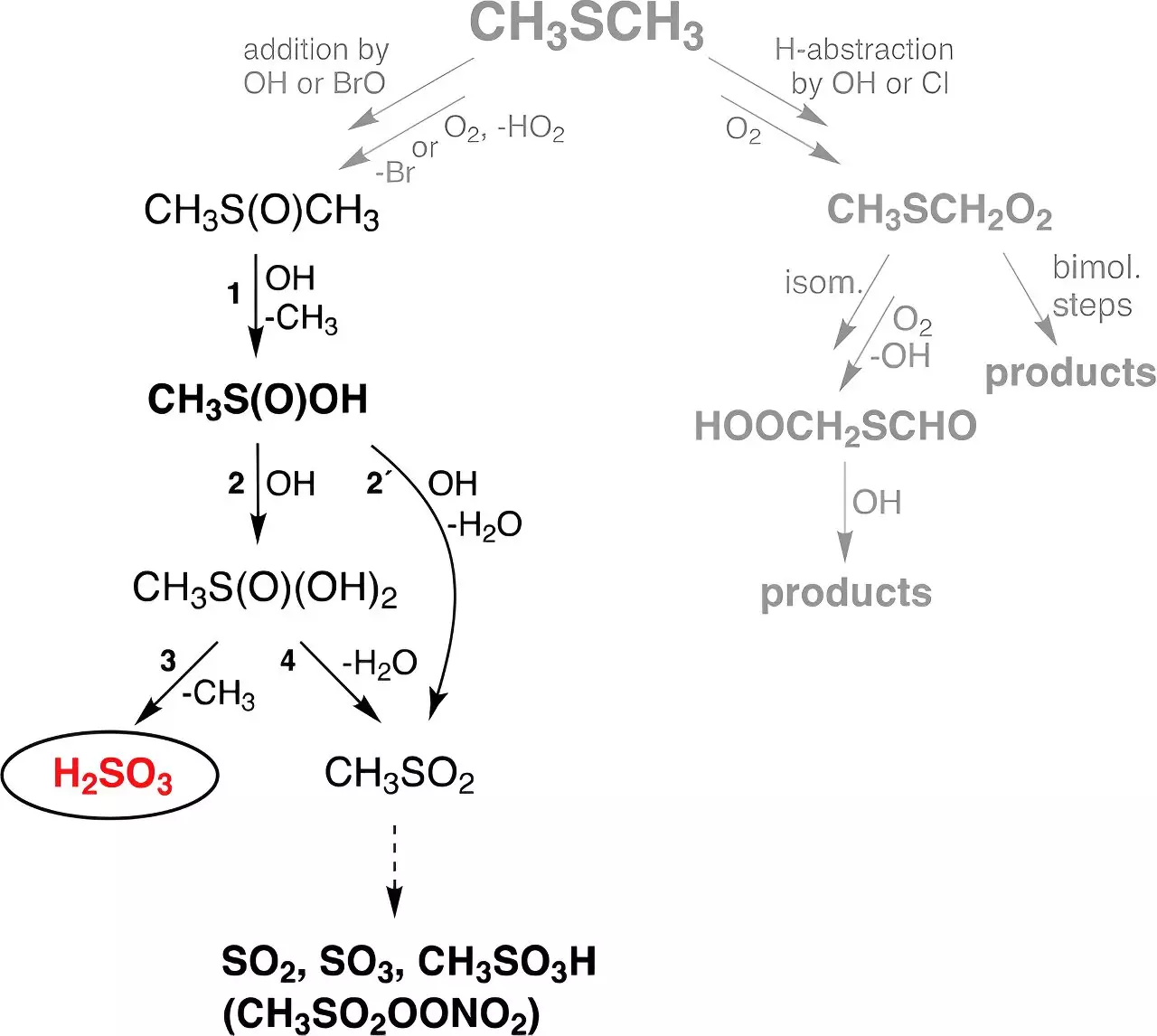
The new findings by the TROPOS team bridge a significant gap in our understanding of atmospheric chemistry. The research led by Dr. Torsten Berndt centers on the theoretical predictions that H2SO3 could form through gas-phase reactions involving hydroxyl radicals (OH) produced mainly from ozone and water molecules in the presence of ultraviolet (UV) radiation. A particularly noteworthy source of these radicals is dimethyl sulfide (DMS), a compound emitted into the atmosphere primarily through biological activities in marine environments, contributing approximately 30 million tonnes of sulphur annually.
In carefully controlled laboratory conditions, researchers demonstrated the formation of H2SO3 in gas phase flow reactors simulating atmospheric conditions. Their experiments revealed that sulfurous acid remains stable for as long as half a minute, unaffected by humidity levels, which raises intriguing possibilities regarding its potential atmospheric lifetime. However, the need for further research is evident as longer atmospheric residence times could not be assessed with the current experimental setups.
Perhaps the most astonishing revelation from the study is the estimated global production rate of H2SO3. Using predictive modeling, the researchers concluded that approximately 8 million tonnes of sulfurous acid are produced each year—a figure that remarkably exceeds the amount generated through direct formation of sulfuric acid (H2SO4) from DMS by a factor of 200. Dr. Andreas Tilgner and Dr. Erik Hoffmann, who contributed to the global modeling aspect of the research, stress the importance of these results in refining our understanding of the atmospheric sulfur cycle.
Despite the excitement surrounding these findings, they also open the door to new questions. The stability of H2SO3 in its gaseous state is a promising discovery, yet uncertainties remain regarding its reactivity with trace gases in the atmosphere, particularly in interactions with water vapor. Dr. Berndt emphasizes that more rigorous and optimized experiments are essential for clarifying the role sulfurous acid plays in atmospheric chemistry.
This study exemplifies the profound impact of advances in analytical chemistry techniques. The researchers employed a high-sensitivity mass spectrometer capable of detecting extremely low concentrations—a mere 10^4 molecules per cubic centimeter. This level of sensitivity allows scientists to discern specific molecules amidst a staggering background of 10^15 other molecules, thereby enhancing our ability to investigate previously challenging chemical processes.
As research methodologies continue to improve, they will undoubtedly pave the way for deeper insights into the complexities of atmospheric chemistry and beyond. Harnessing such innovative tools equips scientists with the means to validate theoretical constructs and explore new reaction pathways, adding a rich layer of understanding to various branches of chemistry.
The discovery and experimental confirmation of sulfurous acid presents a paradigm shift in atmospheric chemistry. Insights gained from this research contribute valuable knowledge to the intricate dance of chemical processes that define the Earth’s atmosphere. As scientists impel further research to understand the full implications of their findings on sulfur cycles and broader atmospheric phenomena, the potential exists not only for additional discoveries but for the refinement of global climate models, thereby reinforcing our commitment to understanding the ever-evolving dynamics of our environment. In time, this work may illuminate the complex interplay of atmospheric constituents, propelling us toward more effective strategies for addressing environmental challenges.

Leave a Reply