As the world increasingly pivots towards a sustainable and carbon-neutral future, the demand for energy storage solutions is surging. This is particularly true in sectors such as electric vehicles (EVs) and large-scale energy storage, where traditional lithium-ion batteries (LiBs) often fall short. The quest for innovative and more efficient batteries has led researchers to explore an alternative: lithium-metal batteries (LMBs). Unlike LiBs, which utilize graphite-based anodes, LMBs employ lithium metal anodes, promising substantial advancements in energy density and charging speeds.
Though LMBs sound promising, they are not without challenges. Current models exhibit significant weaknesses such as high production costs, limited cycling stability, and the notorious formation of lithium dendrites during charge cycles—structures that not only diminish performance but also pose severe safety risks. As a reaction, scientific communities are actively investigating methods to optimize the electrode/electrolyte interface, a crucial component determining a battery’s overall efficiency and safety.
Dendrite Formation: A Barrier to Efficiency
One of the most significant challenges in LMB technology is the formation of lithium dendrites, which occur when lithium ions deposit unevenly during charging. These dendritic structures can penetrate the separator between the anode and cathode, potentially causing short circuits, overheating, and even fires. Addressing this issue is not merely a matter of improving battery design but involves a thorough understanding of the interactions at the electrolyte/electrode interface.
Recent advancements have focused on modifying the electrolyte composition and the electrochemical environment to mitigate dendrite growth. Researchers have turned their attention toward stabilizing the solid-electrolyte interphase (SEI), which is crucial for ensuring the longevity and reliability of lithium-metal batteries. By precisely controlling the interface where lithium ions interact with the electrolyte, it becomes feasible to minimize disruptions that lead to dendrite formation.
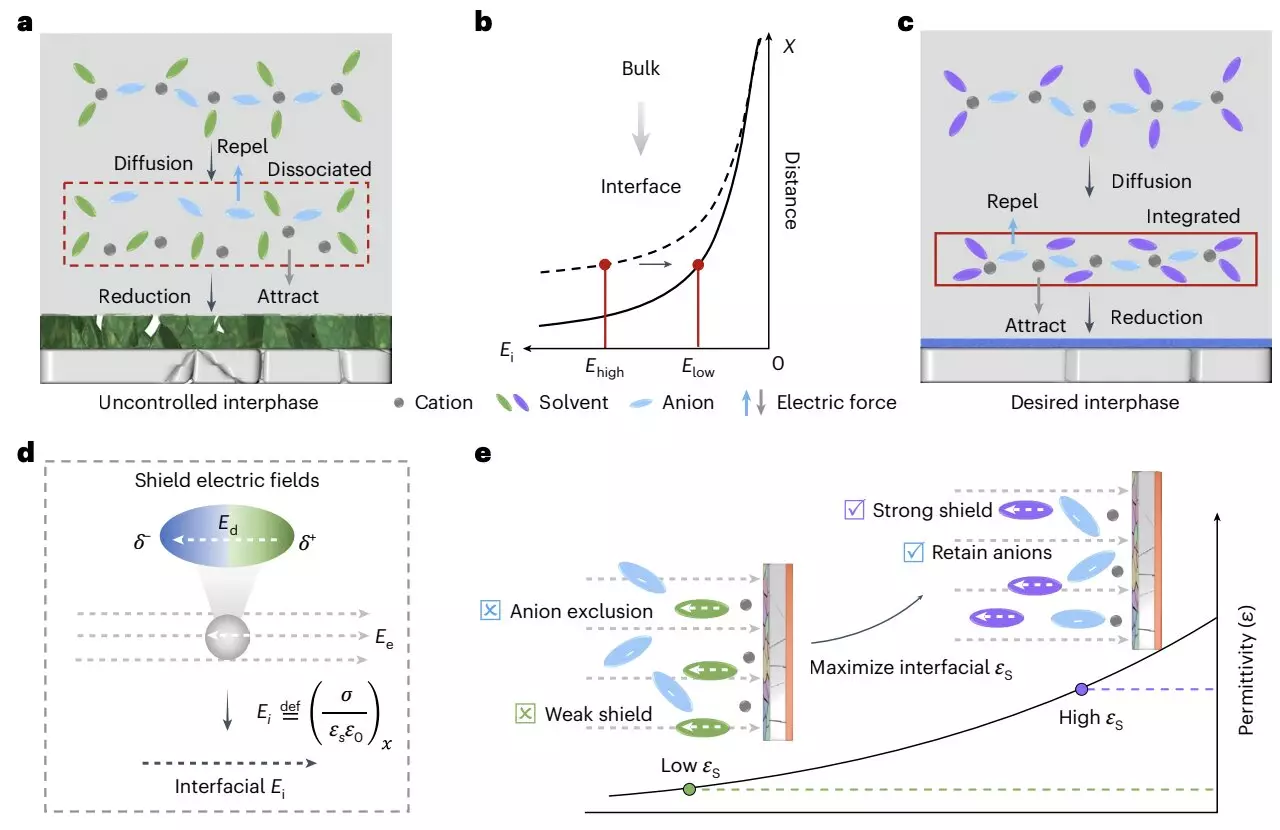
A team of researchers at Zhejiang University and collaborating institutions in China has recently published a groundbreaking study in *Nature Energy* that presents a novel dielectric protocol aimed at enhancing the interface between electrodes and electrolytes in LMBs. The innovative approach emphasizes the importance of the dielectric environment and its influence on the interfacial electric field.
The researchers argue that regulating the dielectric medium can considerably improve the coordination of cation-anion pairs, which is vital for the formation and stability of the SEI. By employing non-solvating solvents with high dielectric constants, the study shows how it is possible to create an anion-rich layer near the electrode surface, promoting anion decomposition at the interface and thereby bolstering interfacial chemistry.
Fan, one of the co-authors of the study, elaborates on the significance of this process. “By adjusting the dielectric environment, we can better protect the cation-anion pairs from dissociation. This offers a more favorable spatial configuration that enhances the electrochemical stability of the battery.”
The implications of this dielectric approach have proven promising in experimental trials. The research team successfully developed an ultra-lean electrolyte configuration and tested it in lithium-metal pouch cells. The resulting energy density reached an impressive 500 Wh/kg—far exceeding the performance of traditional LiBs.
These extraordinary results indicate that the spatial arrangement of ions at the electrode/electrolyte interface can significantly influence both the safety and efficiency of LMBs. The study highlights a well-coordinated interplay between dielectric properties and ionic interactions, providing a new pathway for designing high-performance electrolytes.
Future Directions: Pathway to Enhanced Battery Solutions
The findings from this research not only open new doors for enhancing lithium-metal batteries but also serve as a foundation for further exploration in the field of battery technology. Other research teams could take inspiration from this dielectric-mediated fundamental approach as they work towards developing safer, more efficient electrolytes for LMBs.
As industries increasingly rely on high-energy-density battery solutions, the insights gained from this study may catalyze a significant shift in how future battery systems are designed. The overarching goal remains within reach: to create batteries that do not merely replace their predecessors but leapfrog existing technologies, enabling a new era of electrical energy storage that aligns with global sustainability objectives.
As demand for efficient, sustainable batteries continues to grow, especially for electric vehicles and renewable energy applications, research like this serves to underscore the importance of innovation in overcoming the barriers posed by current battery technologies. The enhanced performance and safety offered by a deeper understanding of the dielectric environment promises to shape the future of energy storage.

Leave a Reply