In the realm of biochemistry, few substances evoke as much intrigue as sphingolipids, a diverse class of lipids with critical roles in cellular function and signaling. The journey into the understanding of these molecules began in the late 19th century with German pathologist Ludwig Thudichum, who identified sphingolipids during his research on brain lipids. He aptly named them after the Sphinx of Greek mythology, hinting at the complexities and challenges they present to researchers. This historical exploration has since led to the identification of several diseases linked to improper sphingolipid metabolism, emphasizing the need for innovative research in this area.
Today, the significance of sphingolipids extends beyond neurodegenerative conditions like Fabry’s disease and Gaucher’s disease. Researchers have identified strong correlations between sphingolipid dysfunction and a range of infectious diseases, including those caused by viruses such as Ebola, COVID-19, and various bacterial pathogens. Notably, the enzyme sphingomyelinase plays a pivotal role in this process by catalyzing the breakdown of sphingomyelin, a type of sphingolipid. The degradation of sphingomyelin is crucial in both viral and bacterial pathogenesis, but until recently, visualizing the activity of sphingomyelinase within biological systems remained an elusive challenge.
Recent advancements in this field have emerged from the collaborative efforts of researchers in Würzburg and Berlin, presenting a novel sphingomyelin derivative aimed at visualizing sphingomyelin metabolism during infections. This groundbreaking study, published in the journal Nature Communications, marks a significant advancement in our understanding of how sphingomyelin metabolism intersects with infectious disease mechanisms. The research originates from the interdisciplinary Research Training Group 2581, which unites chemists, physicists, and biologists in the common goal of elucidating the complex interactions between lipids and pathogen-related processes.
This innovative derivative is described as a trifunctional sphingomyelin, engineered to mimic natural sphingomyelin while incorporating additional functionalities. According to Professor Jürgen Seibel from the Institute of Organic Chemistry at Julius-Maximilians-Universität Würzburg, synthesizing such compounds posed considerable challenges due to the need for their acceptance and integration by biological systems. This intricacy signifies the meticulous design work undertaken by the research team, which ultimately bears the potential to revolutionize infection research.
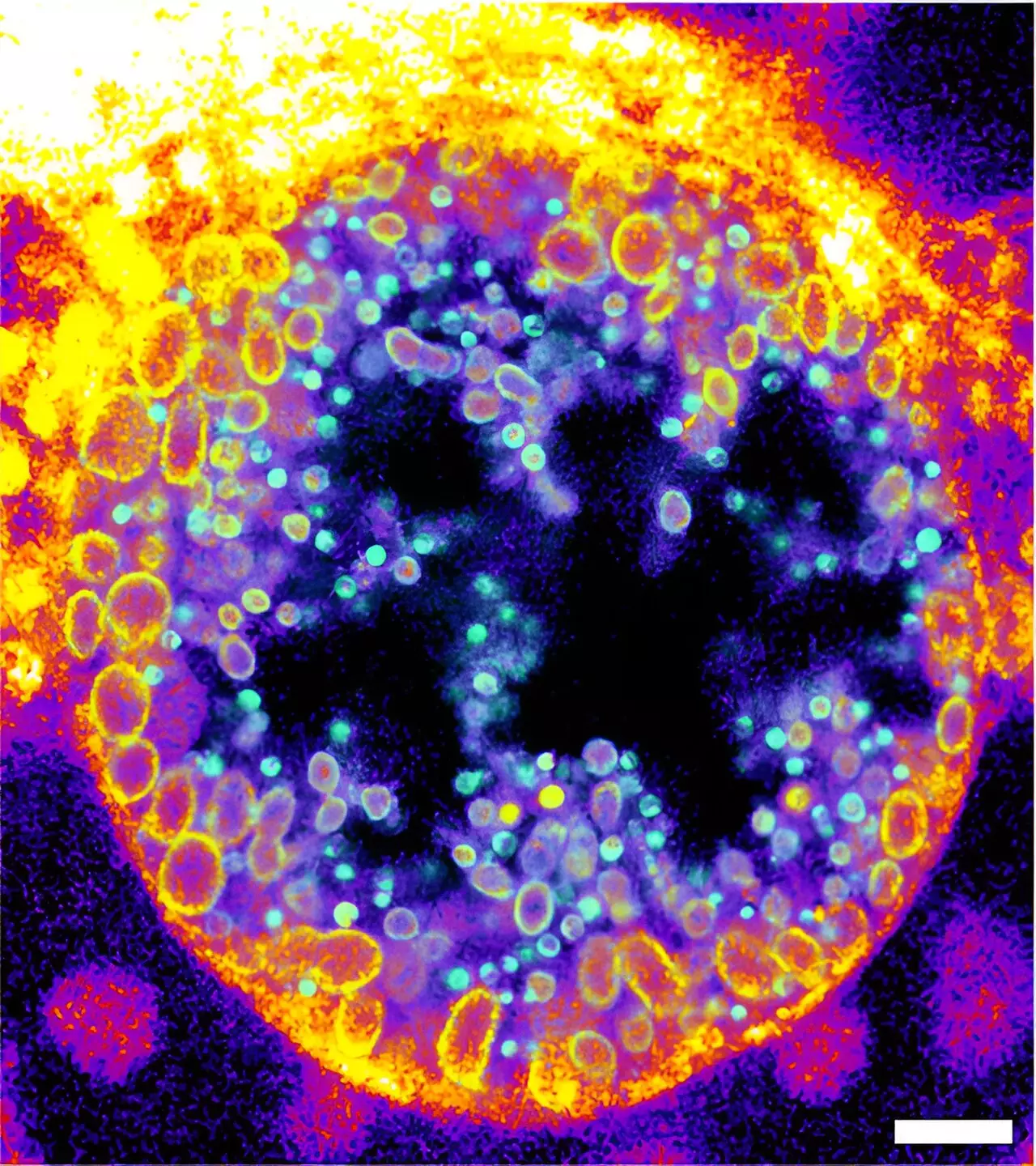
The research team could successfully demonstrate the practical applications of their synthesized sphingomyelin derivatives through innovative methods. They showcased the activity of bacterial sphingomyelinase on human cell surfaces and, crucially, visualized sphingomyelin degradation amid infections with intracellular Chlamydia bacteria. Chlamydia are notorious for causing various infections, particularly in the human genital tract, and are suspected of contributing to cancer development in infected tissues.
The researchers employed cutting-edge techniques, including expansion microscopy and click-chemistry, to observe the dynamics of sphingomyelin during Chlamydia infections. The results revealed that chlamydial inclusions, the compartments formed within host cells, predominantly contained the cleaved forms of trifunctional sphingomyelins. The longitudinal study provided a novel insight into the lifecycle of Chlamydia, showing a quantitative increase in metabolized sphingomyelin as the infectious particles matured.
These findings carry significant implications for infectious disease research and therapeutic development. By enabling the visualization of sphingomyelin degradation within infected cells, researchers can now target specific pathways involved in sphingolipid metabolism. This opens doors to novel therapeutic strategies aimed at combating infections that leverage the metabolic intricacies associated with sphingolipids.
As Professor Seibel aptly states, the introduction of this new chemical tool is poised to become a staple in laboratories globally, facilitating cutting-edge research aimed at unraveling the complexities of sphingomyelin metabolism in various infectious contexts. The marriage of chemistry, biology, and physics in this research underscores the importance of interdisciplinary collaboration in tackling the intricate puzzles posed by sphingolipids and their role in health and disease.
The discovery and application of new sphingomyelin derivatives represent a pivotal step in infection research, enhancing our capacity to visualize and understand sphingomyelin metabolism’s role in infectious diseases. The potential for innovative therapeutic approaches holds promise for addressing some of the most challenging health issues facing society today. Researchers’ dedication to deciphering this complex world of sphingolipids may ultimately lead to significant advancements in treatment and prevention strategies for infectious diseases.

Leave a Reply