The synthesis of organic molecules is an essential function across various industries, from pharmaceuticals to materials science. Traditionally, these chemical reactions are predominantly conducted in liquid phases, which facilitate interactions between substrates and catalysts. However, the reliance on organic solvents, often toxic and environmentally hazardous, poses significant challenges. A notable portion of waste produced during chemical processes arises from these solvents, leading to environmental concerns when not disposed of properly. A team of researchers from the Department of Inorganic and Physical Chemistry (IPC) at the Indian Institute of Science (IISc) has proposed a groundbreaking approach to mitigate this issue through the development of an innovative surfactant derived from agricultural waste.
Organic solvents have long been the backbone of chemical synthesis but their drawbacks are becoming increasingly apparent. More than 80% of waste generated in chemical reactions stems from these solvents, contributing to pollution and requiring stringent disposal methods. Furthermore, many substrates and catalysts are sensitive to moisture, resulting in side reactions that yield undesirable products. The need for a sustainable, efficient solution is evident, especially considering the mounting pressure on industries to adopt greener practices. Recognizing this, the research team at IISc sought to create a bio-based alternative that could effectively facilitate important chemical reactions while minimizing environmental impact.
The researchers found inspiration in cashew nut shell liquid (CNSL), an agricultural by-product produced during the processing of cashew nuts, which India produces in large quantities. This material not only provides a cost-effective raw material source but also embodies the principles of sustainability by repurposing waste. The surfactant they designed, named CNSL-1000-M, effectively bridges the gap between hydrophilic and hydrophobic environments. By chemically combining cardanol—found in CNSL—with m-PEG, a polyether that is hydrophilic, they created a surfactant that exhibits unique properties suitable for micellar catalysis.
At the core of their innovation lies the ability of CNSL-1000-M to self-assemble into micelles when introduced to water. These microscopic structures have a hydrophobic center that encases substances sensitive to water, creating isolated environments for chemical reactions to occur without interference from the aqueous phase. The analogy of a football floating on a body of water aptly describes how substrates can be encapsulated within micelles, allowing for selective chemical transformations to occur in a controlled and protected setting.
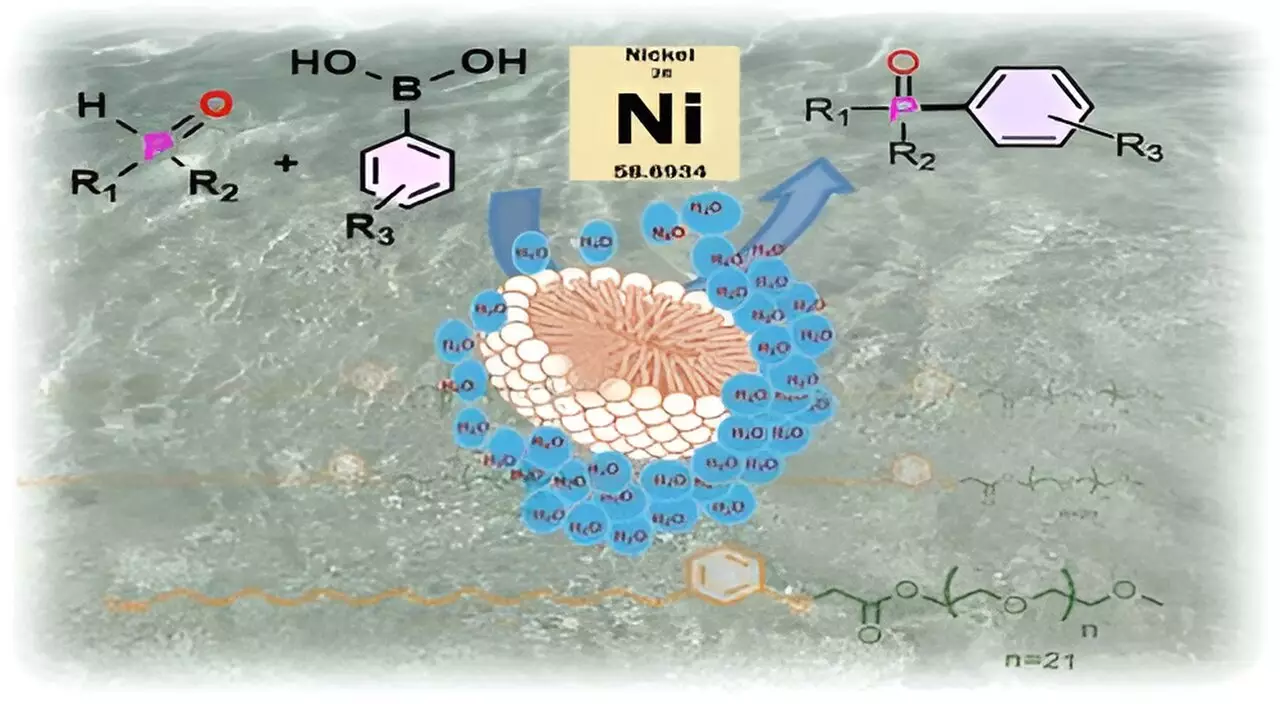
This process not only mimics biological systems but also enhances reaction efficiency. The team utilized CNSL-1000-M to catalyze carbon-phosphorus bond formation, a key mechanism in producing various chemical compounds, including vital pharmaceuticals like the anticancer agent Brigatinib. The results were impressive; the surfactant yielded an 80% increase in product yield when reactions were conducted in water compared to those carried out in traditional organic solvents. Additionally, it demonstrated a 30% improvement over existing surfactants, showcasing its potential as a leading alternative in the field.
The avenues for utilizing CNSL-1000-M extend beyond increased yields. This novel surfactant can replace costly palladium catalysts with more affordable nickel complexes, thereby reducing the overall costs associated with chemical processes. Moreover, the ability to facilitate reactions at lower temperatures further exemplifies the potential economic and environmental benefits of this research.
The researchers’ vision is ambitious and clear: to collaborate with the industry to transition chemical synthesis methods from hazardous organic solvents to more sustainable aqueous micellar technologies. By focusing on micellar chemistry and its industrial applicability, they aim to foster greener practices across various sectors.
The work being carried out by the IISc team highlights an important shift toward sustainable chemistry. By leveraging agricultural waste to develop effective catalysts, they are proactively addressing both economic and environmental challenges in chemical synthesis. The future of green chemistry looks promising, with innovations like CNSL-1000-M paving the way for cleaner, safer, and more efficient industrial practices.

Leave a Reply