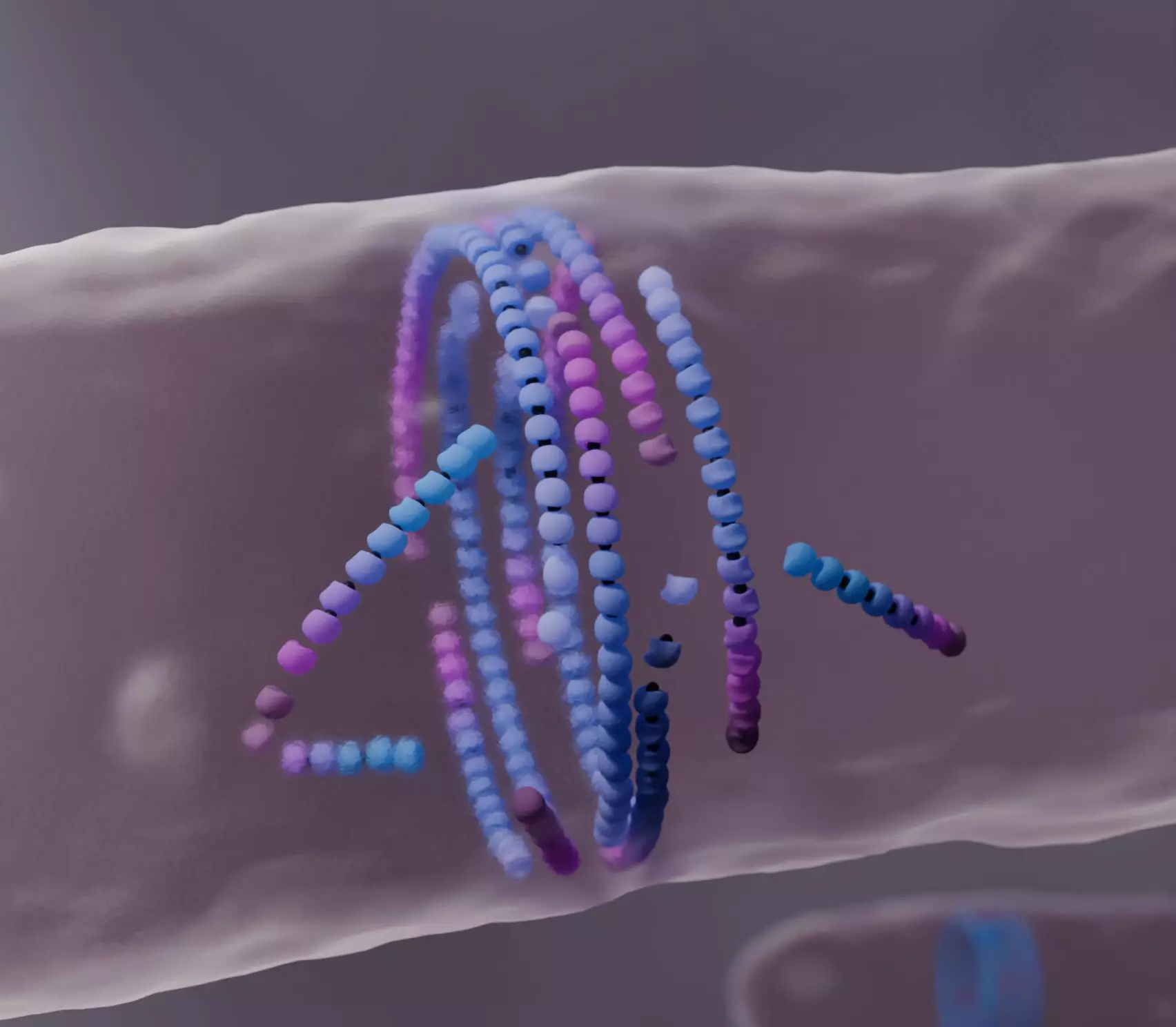
The phenomenon of self-organization is one of the most intriguing characteristics of life. In essence, it encompasses the ability of certain systems to spontaneously arrange their components in a structured manner. A recent study conducted by researchers from the Institute of Science and Technology Austria (ISTA) has unveiled a previously unknown mechanism pertaining to bacterial cell division. This mechanism operates under the metaphor of “dying to align,” wherein misaligned filaments of FtsZ—the protein responsible for bacterial cell division—destabilize and reassemble to form a centralized ring structure during cell division. The implications of these findings extend beyond the realm of biological research, potentially influencing the development of synthetic self-healing materials.
A dense web of questions permeates the realm of self-organization in biological systems. How do these systems discern the precise moments to assemble and disassemble? What underlies the laws of nature that govern such processes? The work spearheaded by Professor Anđela Šarić and her team centers on a protein known as FtsZ, which plays a critical role in bacterial cell division. FtsZ self-assembles into a ring structure that facilitates the formation of a new cellular wall, ultimately partitioning the daughter cells. While extensive studies have elucidated the role of this protein, the fundamental physical interactions involved in its self-assembly have remained curiously elusive.
To demystify the biological processes at play, Šarić and her colleagues developed a sophisticated computational model capable of simulating the interactions between FtsZ subunits. Unlike previous models that merely focused on self-propulsion, the team recognized the necessity of representing molecular behaviors through the concept of “treadmilling.” This term signifies the cyclical nature of filament growth and shrinkage, where subunits are continually added at one end of the filament while removed from the other.
The breakthroughs emerged from observations made during simulations. Instead of moving uniformly, the misaligned FtsZ filaments exhibited a tendency to “die”—dissolve entirely upon encountering an obstacle. Through this phenomenon, the remaining filaments could align themselves more effectively, forming a robust and structured division ring essential for cell replication.
Critical to the research’s advancement was the fruitful collaboration between computational modeling and experimental studies. Upon presenting their findings at the “Physics Meets Biology” conference, the ISTA team connected with experimentalists from the University of Warwick and other institutions. Notably, scientists led by Seamus Holden highlighted evidence supporting the necessity for filament “death” and subsequent rebirth in forming the FtsZ division ring. The real-time imaging conducted by Holden’s team of Bacillus subtilis was indicative of the performance predicted by the computational model.
Furthermore, the collaboration bore fruit when Šarić’s team partnered with Martin Loose’s research group, which had been exploring FtsZ interactions in vitro. The experimental results aligned remarkably well with the computational predictions, providing a solid foundation for the hypothesized mechanism of self-organization through filament turnover.
The study reinforces a shift in how scientists perceive active matter, specifically in relation to its capacity for self-organization through turnover rather than mobility. Šarić notes that this work has significant implications for creating synthetic self-healing materials and even synthetic cells. By understanding the underlying principles of molecular assembly and disassembly, researchers may develop materials that mimic these natural processes, fostering resilience and adaptability in man-made structures.
Looking ahead, Šarić and her team aim to delve deeper into the specifics of how the FtsZ division ring contributes to cell wall construction. This ongoing exploration into the frontiers of biology not only holds the promise of groundbreaking advancements in materials science but also offers insights into the very processes that constitute life.
The research presented by the Šarić group serves as a poignant reminder of the complexity and sophistication inherent in biological systems. As science ventures further into understanding the multifaceted nature of self-organization, the potential applications of such knowledge continue to expand. From the intricacies of bacterial cell division to the potential for innovative synthetic materials, the exploration of how matter—living and non-living—interacts, aligns, and adapts remains an essential and exciting frontier in contemporary scientific inquiry.

Leave a Reply