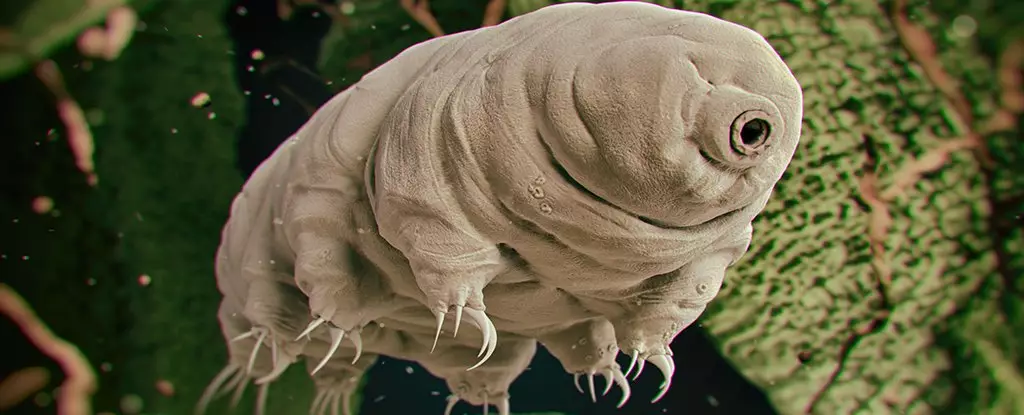
Tardigrades, often affectionately referred to as “water bears” or “moss piglets,” are microscopic creatures renowned for their astonishing survival abilities. These tiny, eight-legged animals can withstand extreme conditions that would obliterate most known life forms, including exorbitant temperatures, immense pressures, and, notably, high doses of radiation. This resilience has captured the attention of researchers, who are now exploring the potential of tardigrades’ unique biochemistry to revolutionize cancer treatments, particularly radiotherapy.
Radiation therapy is a common cancer treatment that targets aberrant tumor cells with high-energy radiation to destroy or inhibit their growth. However, this beneficial application is not without significant collateral damage; healthy cells also suffer the consequences, experiencing DNA breaks that lead to detrimental side effects. These range from mild discomfort, such as oral sores, to severe conditions requiring hospitalization. Dr. James Byrnes, a radiation oncologist at the University of Iowa, emphasizes the extreme discomfort and complications that some patients endure, highlighting a crucial need for advancements in therapy that can differentiate between healthy and cancerous tissues.
The Discovery of Damage Suppressor Protein
What is it that allows tardigrades to execute such remarkable feats of endurance against radiation? Their secret lies in a remarkable protein known as Dsup, or ‘damage suppressing’ protein. Since its discovery in 2016, Dsup has intrigued the scientific community, demonstrating the capability to reduce radiation-induced DNA damage by nearly 40% when expressed in human cells. Researchers, led by Ameya Kirtane from Harvard Medical School and Jianling Bi from the University of Iowa, are now aiming to harness the properties of Dsup to protect healthy cells during radiotherapy.
Pioneering the Use of Messenger RNA
Kirtane and his team have devised an innovative strategy by using messenger RNA (mRNA) to deliver the Dsup protein into cells rather than integrating it directly into the DNA. This temporary expression method provides a safety advantage—since mRNA doesn’t risk permanent alteration of the cells’ genome, it minimizes potential adverse effects associated with genetic modifications. The researchers encapsulated the mRNA within advanced polymer-lipid nanoparticles tailored for specific tissues, enhancing the likelihood of effective delivery. This dual approach not only maximizes the delivery potency but also ensures that the protective influences of Dsup do not extend to tumor cells.
To evaluate the efficacy of this groundbreaking approach, the team conducted experimental trials on mice. The subjects were divided into groups that either received the Dsup-encoding mRNA before radiation exposure or were left untreated for comparison. Notably, mice immunized with Dsup demonstrated significantly reduced double-stranded DNA breaks following radiation, with one group experiencing a relative reduction of about 50%. Intriguingly, while the mRNA treatment did not seem to impact tumor size, it nonetheless established a vital foundation for future human studies.
Looking Toward the Future
While this research undoubtedly points to promising pathways in the realm of cancer treatment, it’s crucial to acknowledge limitations. The small sample sizes and animal-based studies merely provide initial insights, and the translation of these findings to human physiology remains uncertain. However, the results mark a significant step toward enhancing patient safety and therapeutic effectiveness in radiotherapy.
The potential applications of Dsup mRNA delivery extend beyond just aiding radiotherapy. The authors speculate that it could play a role in protecting normal tissues from the adverse effects of various DNA-damaging chemotherapeutic agents, coping with tissue degeneration, and addressing issues associated with chromosomal instability.
The study of tardigrades has not only expanded our understanding of resilience in extreme environments but has also opened new avenues for medical innovation. As researchers continue probing the depths of this miniature marvel’s biology, the hope is that the mechanisms driving its survival can be translated into significantly improved cancer therapies. Through careful exploration and experimentation, scientists may soon diminish the painful side effects of radiation therapy, leading to a more humane outlook on cancer treatment. The journey from the microscopic worlds of tardigrades to the complex biology of human cells could redefine cancer therapy for future patients—a testament to the profound connections present in nature.

Leave a Reply