Z-alkenes, featuring a carbon-carbon double bond with substituents aligned on the same side, play an essential role in organic chemistry and biological systems. Their structural uniqueness not only influences reactivity and interaction with biological molecules, but it also renders them pivotal for the development of complex organic compounds, including pharmaceuticals. Understanding how to efficiently synthesize Z-alkenes is crucial, especially given their prevalence and significance in various chemical applications.
Traditionally, the synthesis of Z-alkenes has posed various challenges, often proving to be inefficient and time-consuming when using thermodynamic approaches. Researchers are continually exploring innovative methods to overcome these synthesis hurdles, marking a crucial area of study in organic chemistry.
Photoisomerization offers a beacon of hope for the synthesis of Z-alkenes, leveraging light energy to convert E-alkenes into their Z counterparts. This innovative approach alters the configuration of molecules in a way that many conventional methods cannot, elevating its status in the field. The ability to directly transform E-alkenes, which are more readily accessible through traditional synthetic routes, into Z-alkenes through photoisomerization underscores the significance of harnessing photochemical processes.
One particularly intriguing aspect of photoisomerization is its versatility, allowing applications ranging from organic chemistry to the development of advanced materials in polymer science and medicinal chemistry. The ongoing challenge, however, has been to streamline the photoisomerization process for greater efficiency and feasibility in broader applications.
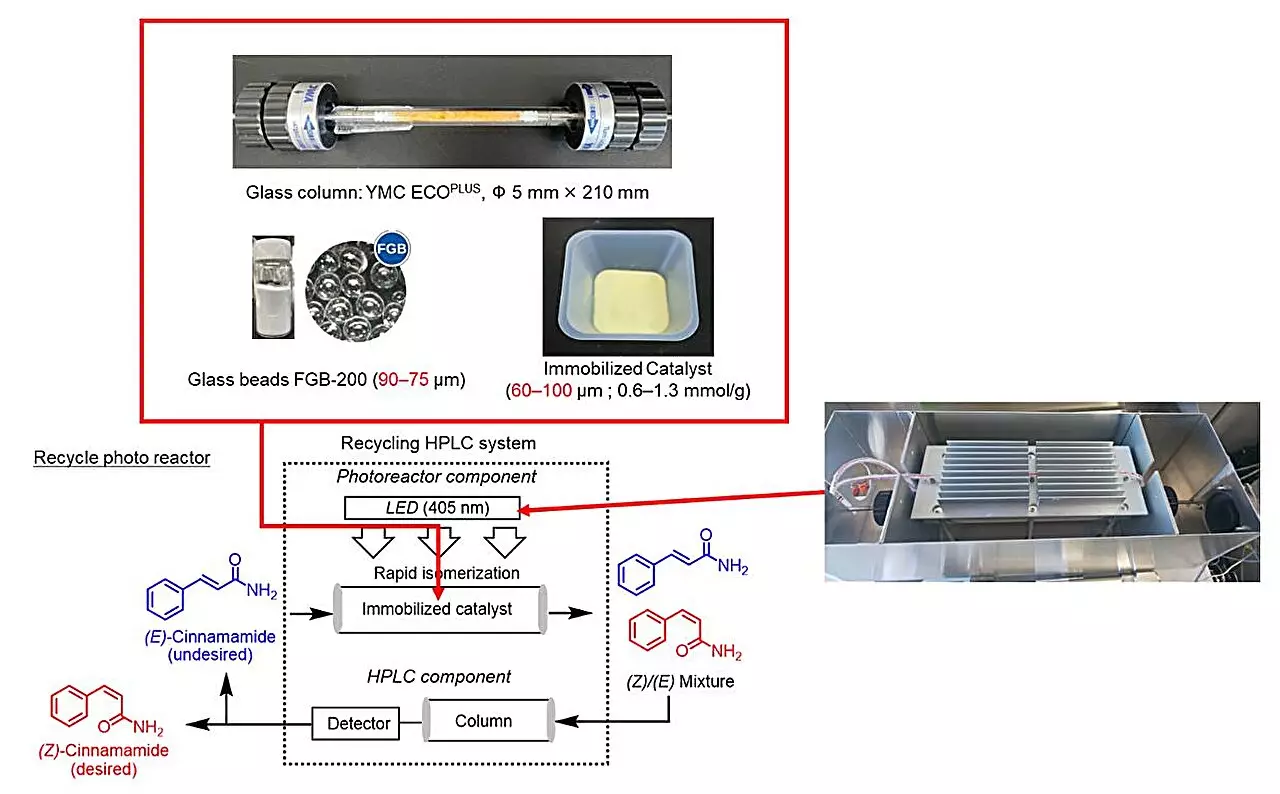
Recent strides in the research of Z-alkene synthesis have emerged from a notable collaboration led by Professor Hideyo Takahashi of the Faculty of Pharmaceutical Sciences at Tokyo University of Science. Their recent study, published in The Journal of Organic Chemistry, details a novel recycling photoreactor that capitalizes on the principles of deracemization; an innovative system that previously demonstrated success in purifying chiral molecules.
The research team adeptly adapted this closed-loop recycling photoreactor for the photoisomerization of E-cinnamamides into Z-cinnamamides. This ingenious approach not only facilitates the process of isomerization but also minimizes waste and maximizes sustainability, presenting a remarkable shift toward eco-friendly synthetic practices.
Crucial to successful photoisomerization is the choice of photosensitizer, a material that absorbs light and catalyzes the transformation of E- to Z-alkenes. The team’s diligent work involved screening various commercially available photosensitizers, ultimately identifying thioxanthone as the most effective candidate due to its exceptional ability to promote rapid photoisomerization.
By cleverly immobilizing thioxanthone on modified silica gel with functional amide groups, the researchers not only ensured stability by reducing leaching during reactions, but they also sparked a remarkable catalytic efficiency compared to thioxanthone in its soluble form. This finding is particularly striking given that solid-phase reactions have historically been hindered by slower kinetics than their liquid-phase counterparts. The introduction of appropriate functional groups played a pivotal role in this enhancement, propelling the team to explore the catalytic capacities of various other photosensitizers.
The standout feature of the developed recycling photoreactor is its ability to conduct continuous reactions across multiple cycles while maintaining high yields of desired Z-alkenes. After conducting a series of experiments, the researchers observed optimal outcomes after four to ten cycles, a testament to the system’s efficiency and sustainability.
Professor Takahashi’s assertion that this approach offers a promising alternative for producing Z-alkenes signals a monumental shift toward greener synthesis methods. Not only does this technique diminish reliance on hazardous solvents or excessive waste, but it aligns with the ongoing global discourse on sustainability in chemical manufacturing, ultimately fostering a more responsible approach to pharmaceutical development.
The intersection of innovative research, environmental stewardship, and high-performance chemistry showcased in this study positions it as a landmark achievement in organic chemistry and demonstrates the immense potential for future advances in sustainable practices. As we navigate an ever-evolving chemical landscape, such research paves the way for a more connected understanding of sustainability in the synthesis of essential organic compounds.

Leave a Reply