Recent research spearheaded by Professor Jaeheung Cho and his team at the Department of Chemistry at UNIST has unveiled critical insights into the interaction between cobalt(III)-based metal complexes and nitrile compounds. Featured in the Journal of the American Chemical Society, this study lays the groundwork for novel approaches in drug development by elucidating the intricacies of nitrile activation through biomimetic processes. Nitriles are pivotal in various pharmaceutical applications, yet their inherent reactivity has posed considerable challenges, making this investigation particularly significant.
The examination of nitrile activation mechanisms in this research underscores the pivotal role of metal spin states. These properties are not mere academic curiosities; they directly dictate the efficiency and output of chemical reactions. The research illustrates that minor alterations in the structural attributes of cobalt reagents can lead to substantial shifts in the reaction’s kinetics and by-products. Such revelations stress the importance of precise fine-tuning in chemical design, which could serve as a guiding principle for future organic synthesis and drug formulation initiatives.
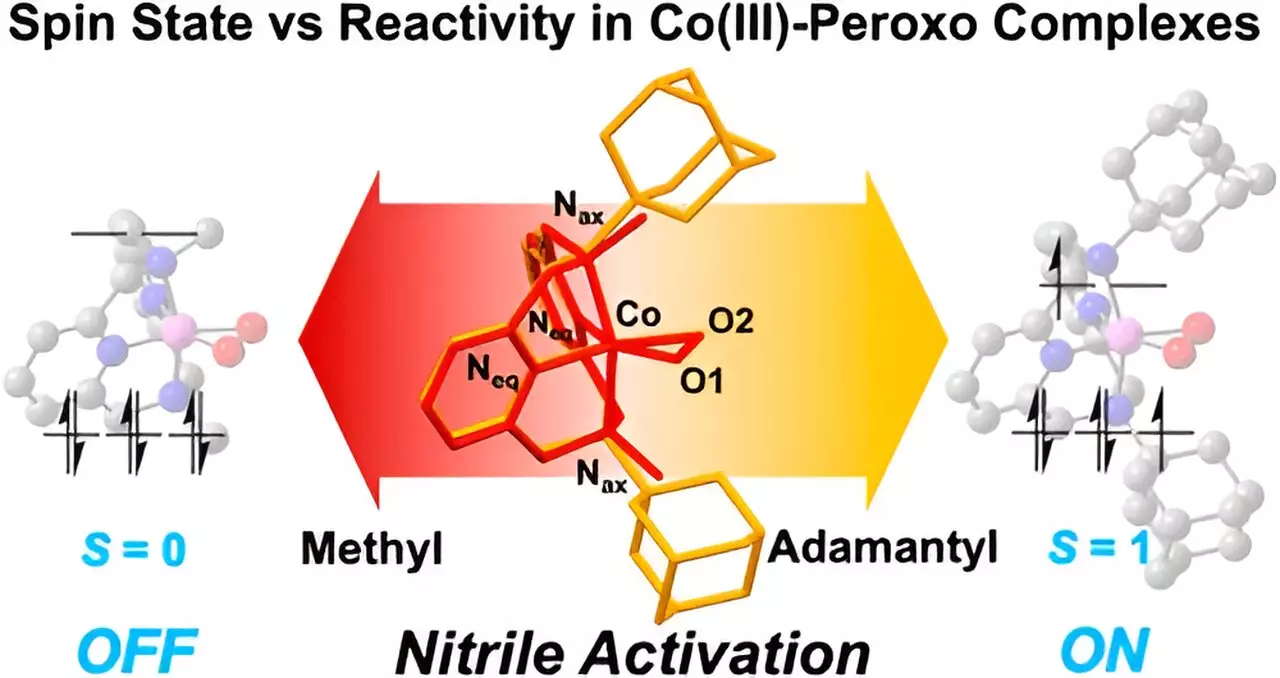
Utilizing the Macrocyclic Pyridinophane System, the research team delved into how the size of functional groups affects nitrile activation. The differentiation between larger adamantyl groups and their smaller methyl counterparts reveals a critical evolutionary aspect in reaction dynamics. Specifically, the study found that the larger adamantyl functional groups facilitated more vigorous nitrile activation than the smaller groups, establishing a clear correlation between structural dimensions and reactivity profiles.
The team successfully synthesized cobalt(III)-peroxo species exhibiting various spin states, demonstrating a distinct link between these configurations and nitrile reactivity. Their findings suggest that cobalt(III)-peroxo complexes can effectively react with nitriles at ambient temperatures to yield compounds that exhibit potential as anticancer agents. This insight not only broadens the scope of how we perceive cobalt complexes but also opens avenues for developing targeted therapies that could address significant medical needs.
The collaborative efforts of Professor Cho’s team delineate a new frontier in the understanding of cobalt(III) chemistry, particularly concerning its interactions with nitriles. These developments present promising implications for the design and creation of new therapeutic agents that may play crucial roles in addressing modern health challenges. As research continues to evolve, the findings from this study will likely encourage further exploration into metal-based complexes in pharmaceutical chemistry, promoting innovative solutions in drug discovery and development.

Leave a Reply