Transcranial focused ultrasound (tFUS) is redefining how we approach the treatment of neurological disorders through its non-invasive methodology. By utilizing high-frequency sound waves directed at specific brain areas, tFUS presents a potential solution for conditions like drug-resistant epilepsy, allowing for localized treatment without the need for surgical methods. Recent advancements by a collaborative team at Sungkyunkwan University, the Institute for Basic Science, and the Korea Institute of Science and Technology have introduced a pioneering sensor designed for this purpose, offering hope for patients seeking alternative therapies for debilitating conditions.
Challenges Faced by Existing Technologies
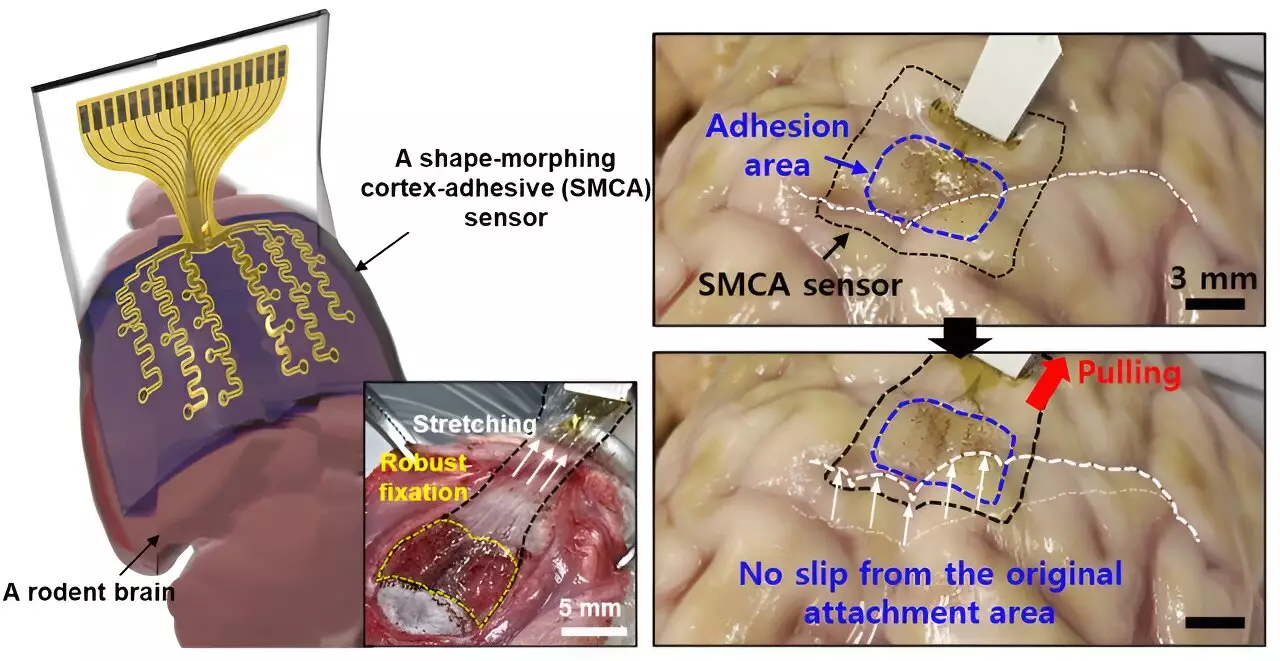
The development of brain sensors that accurately record neural signals has intensified over recent years, yet numerous challenges remain. Donghee Son, the lead researcher behind the new sensor, acknowledges that prior versions were unable to conform adequately to the brain’s intricate topography, thus hampering the effectiveness of treatments. “Previous devices struggled with accurate measurement due to their inability to grip tightly to the brain’s complex surfaces,” Son remarked. This limitation not only obstructed detailed analyses of brain lesions but also rendered real-time monitoring and response to neural activity ineffective.
The existing sensors, while thinner and lighter, often faced issues like detachment during the subtle pulsations of cerebral fluid or head movements. Such shortcomings diminish their practical applicability in medical environments where sustained, accurate readings are essential for effective treatment delivery.
A Revolutionary Sensor Design
In response to these limitations, Son and his colleagues engineered an innovative sensor, the ECoG, which offers superior adhesion to the varied curvature of brain tissue. This advancement is instrumental in ensuring the sensor does not just track brain signals but does so over extended periods of time without the noise interference commonly produced by movements. “The evolution of this sensor allows for a consistent and reliable collection of data, enabling medical practitioners to perform targeted therapeutic interventions,” states Son. The tight adhesion achieved by the ECoG ensures minimal disruption, improving the potential efficacy of treatments aimed at conditions like epilepsy.
The ECoG functions optimally thanks to its unique structure, which comprises three distinct layers. These include a hydrogel layer for tissue bonding, a self-healing polymer that adapts to various surfaces, and a flexible layer embedded with gold electrodes. The combination of these elements prompts a robust attachment and allows the device to interact harmoniously with the brain’s surface, significantly enhancing the accuracy of neural readings.
One of the key advancements with the ECoG is its capability to minimize noise during ultrasound applications, which historically hindered real-time brain monitoring. By providing a clearer signal, the sensor not only benefits the immediate treatment of epilepsy but also lays the groundwork for more personalized approaches. The variability of individual brain conditions requires tailored treatment strategies, a necessity that the ECoG’s noise reduction facilitates. “With reduced external disturbances, we can achieve more precise monitoring and stimulation, enabling a leap toward personalized medicine in the realm of neurology,” Son explains.
The significance of personalizing treatment cannot be overstated, particularly in fields like epilepsy management where variations in response to traditional therapies can be profound. By tailoring ultrasound stimulation based on real-time feedback from the ECoG, clinicians have the potential to enhance treatment efficacy dramatically.
Future Directions and Broader Applications
The promising results from initial tests on living rodents provide a glimpse into the future applicability of the ECoG sensor. As the researchers aim to scale the device into a high-density array, there is a clear trajectory toward addressing more complex neurological disorders. “The goal is to enhance our understanding and responses to brain signaling, paving the way for advanced prosthetics and treatments for other neurological conditions,” enthuses Son.
Meanwhile, plans to increase electrode density for finer brain signal mapping hint at broader implications for research and clinical practice alike. As these technologies evolve, there is palpable excitement surrounding their potential to transform how we diagnose and manage disorders of the nervous system.
The development of the shape-morphing, cortex-adhesive brain sensor represents a significant leap forward in brain research and treatment. These sensors not only provide robust solutions to longstanding challenges faced in the field but also embody the promise of personalized medicine, potentially offering better management of neurological disorders such as epilepsy. With further research and clinical testing, we may soon witness a transformation in neurological care, profoundly impacting countless lives.

Leave a Reply