The battle against cancer is fundamentally a race against the unchecked proliferation of malignant cells. Halting this relentless reproduction hinges on a profound understanding of the proteins that sustain the survival of these malignant cells. Recent efforts have shown that protein profiling is pivotal in identifying specific proteins—which are potential targets for future therapeutics. However, traditional methods have produced limited insights, often failing to identify all relevant protein targets due to inadequate detail. Now, a novel approach that integrates dual strategies of protein analysis has illuminated over 300 small molecule-reactive cancer proteins and their binding sites, opening new avenues for the development of precision cancer treatments.
The Innovation Behind Dual Protein Analysis
Researchers at Scripps Research have initiated a breakthrough by combining two distinct methods of activity-based protein profiling (ABPP), a technique recognized for capturing protein functionalities on a comprehensive scale. As articulated by Dr. Benjamin Cravatt, one of the lead authors of the study published in *Nature Chemistry*, this composite methodology not only provided a broad overview of protein interactions with small molecules but also specified the precise locations of such interactions.
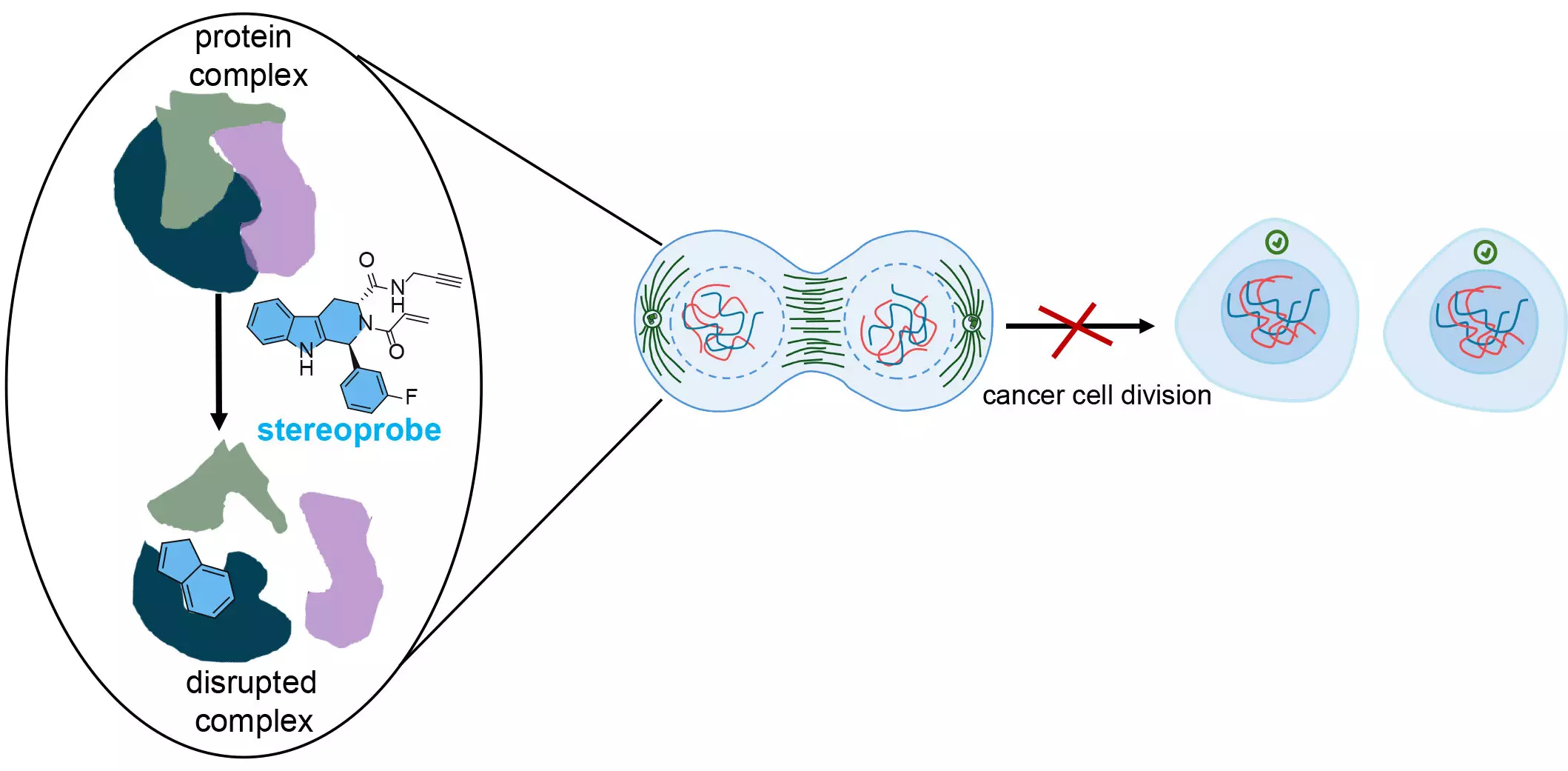
The dual approach employed by the Scripps team engaged a library of stereoprobes—chemicals designed to bind selectively to proteins chemically. These stereoprobes were crafted with innovative features that often remain underrepresented in typical drug discovery compounds, significantly enhancing the group’s capacity to unveil novel biological insights. Co-senior author Dr. Bruno Melillo emphasized that this deliberate design strategy could lead to substantial advancements in both biological understanding and therapeutic efficacy.
A central theme of the research pertained to the amino acid cysteine, an essential building block often present in cancer-related proteins. The stereoprobes were electrophilic, meaning they were tailored to form irreversible bonds with cysteine residues. This specific focus arose from cysteine’s unique properties as the most nucleophilic amino acid, facilitating critical structural bonds in proteins. By interrupting these bonds, the stereoprobes can disrupt the normal functionality of proteins, triggering malfunction that stymies cancer cell growth.
Lead researcher Dr. Evert Njomen elaborated on the significance of targeting cysteine, underscoring the potential of these compounds to inhibit vital interactions within cancer cells. As the stereoprobes engage with critical protein sites, they effectively obstruct necessary interactions, thereby halting cellular division—a crucial step in curtailing tumor growth.
Precision Targeting: The Future of Cancer Therapies
The findings of this study reveal that the dual-profiling strategy not only aids in identifying an extensive array of protein targets but also dramatically enhances the fidelity of the binding information extracted. Prior to this work, each of the traditional single methods had its limitations, leading to a substantial number of missed targets in the protein landscape. Through advancements made in this dual approach, researchers now possess a more refined toolkit for understanding and manipulating the molecular basis of cancer cells.
The key takeaway is that by highlighting specific protein binding pockets essential for cancer cell survival, the potential for developing targeted therapeutic interventions increases exponentially. The ability to target distinct phases in the cell cycle could allow for the creation of novel drugs designed to isolate and neutralize malignant cells, rendering them detectable and vulnerable to the immune system.
While the primary focus of the Scripps Research team’s study is on cancer, their methodologies hold promise for broader applications in other diseases. Dr. Njomen expressed aspirations to construct new libraries of stereoprobes aimed at probing protein activities in various inflammatory disorders and other pathologies. Given that numerous proteins have been implicated in a range of diseases, extending the capabilities of stereoprobes could revolutionize therapeutic exploration across diverse medical fields.
The work undertaken by the team at Scripps Research signifies a transformative moment in cancer research and precise protein profiling. By leveraging innovative approaches to protein analysis, they have not only anticipated future strategies for cancer treatment but perhaps also carved pathways for tackling other significant health challenges. As the landscape of protein-targeted therapies evolves, the hope remains that these unprecedented insights lead to breakthroughs that mitigate the impact of cancer and beyond, fostering a healthier future for humanity.

Leave a Reply