The quest for enhanced energy storage solutions has intensified in recent years, positioning solid-state batteries as a potential revolution in the field of electrochemistry. Distinguished from conventional lithium-ion batteries, solid-state designs promise greater safety, higher energy densities, and improved longevity. At the heart of these advancements lies the critical role of lithium and sodium metal anodes. Their unique properties could significantly elevate performance, provided that researchers surmount the challenges inherent to their use.
Understanding the microstructure of these alkali metals is key to unlocking their potential within the battery realm. The surface interactions and reactive nature of lithium and sodium complicate their utilization in batteries. Typically, these metals quickly form an insulating layer when exposed to operational environments, obstructing both analysis and effectiveness. However, recent efforts from a collaborative team including Justus Liebig University Giessen (JLU), alongside partners from the United States and Canada, have yielded promising results in elucidating this microstructure.
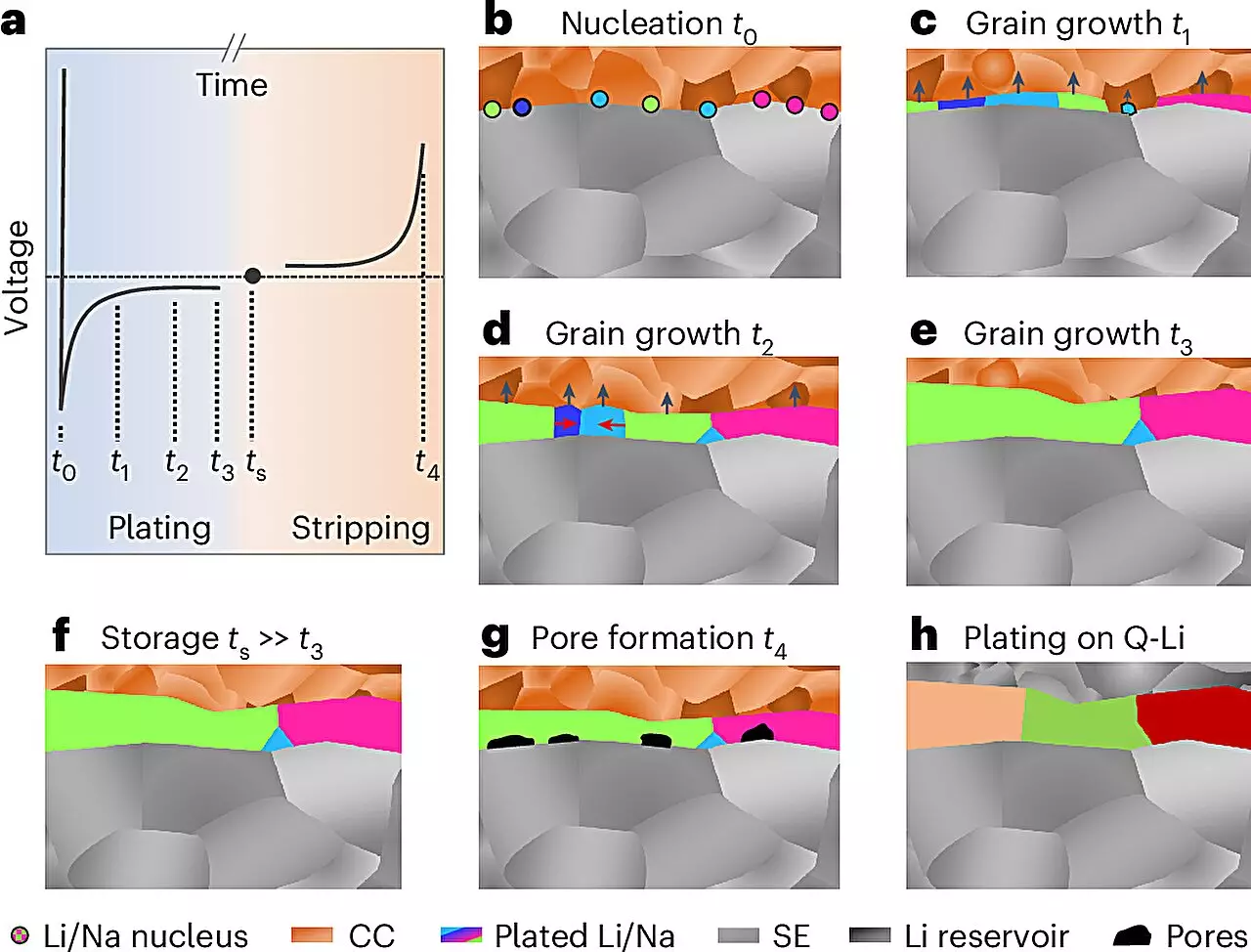
For the first time, a groundbreaking method has been developed to analyze the microstructure of electrochemically deposited lithium and sodium. Under the leadership of Professor Dr. Jürgen Janek from JLU’s Institute of Physical Chemistry, this team demonstrated an innovative series of preparation and analysis techniques that utilized low temperatures and inert gas conditions. This careful approach culminated in the application of electron backscatter diffraction, a powerful imaging technique capable of revealing details of metal layers extending up to 100 micrometers thick.
This landmark study, the findings of which were recently published in *Nature Materials*, highlights how grain size and structural characteristics of the metal layers can drastically influence electrochemical performance. Janek remarked on the unexpected grain size results, which provide deeper insights into the underlying growth mechanisms. Such revelations are crucial for optimizing battery designs that can enhance both charge and discharge cycles, thus aligning with the ambitions of the POLiS excellence cluster focused on alternative energy storage systems.
Microstructure—the arrangement and size of grains within the metal—has profound implications on the performance and stability of battery electrodes. For conventional metals, knowledge about this structure has allowed for targeted improvements in their applications. Yet, until now, alkali metals like lithium and sodium have remained enigmatic. The new findings from JLU and its partners aim to bridge this knowledge gap.
Lithium and sodium’s propensity for dendritic growth during charging is one of the major challenges facing battery researchers. These microscopic structures can lead to short circuits, reducing efficiency and safety. Ideally, it is crucial that lithium or sodium metal only forms during initial charging, mitigating the risks involved with handling alkali metal foils and improving battery stability.
The pathway to more effective solid-state batteries hinges on addressing the inherent challenges posed by lithium and sodium metal electrodes. The recent research breakthroughs signify an important step forward, offering a framework for future investigations into these potent materials. The control and understanding of their microstructures could open the door to a new generation of batteries that integrate the benefits of solid-state chemistry with the high performance typically expected of lithium and sodium metal.
Moreover, Janek’s collaboration with researchers in Santa Barbara and Waterloo has emphasized the power of interdisciplinary teamwork. This approach has proven pivotal in overcoming the historical difficulties associated with imaging and analyzing alkali metals. The precision required in cutting and cross-sectioning these materials for analysis exemplifies the necessity for rigorous methodologies in battery research.
The advancements made through the collaborative research efforts culminate in a clearer understanding of lithium and sodium as potential candidates for high-performance solid-state batteries. As researchers continue to navigate the complexities associated with these materials, the insights gathered will likely shape the future of energy storage technologies.
The success of the outlined method not only enhances scientific comprehension but also lays the groundwork for the practical application of sodium metal in battery technology, potentially revolutionizing the energy landscape. As the world shifts towards more sustainable energy solutions, breakthroughs in solid-state battery technology will become increasingly critical, and this recent research represents a substantial stride in that direction.

Leave a Reply