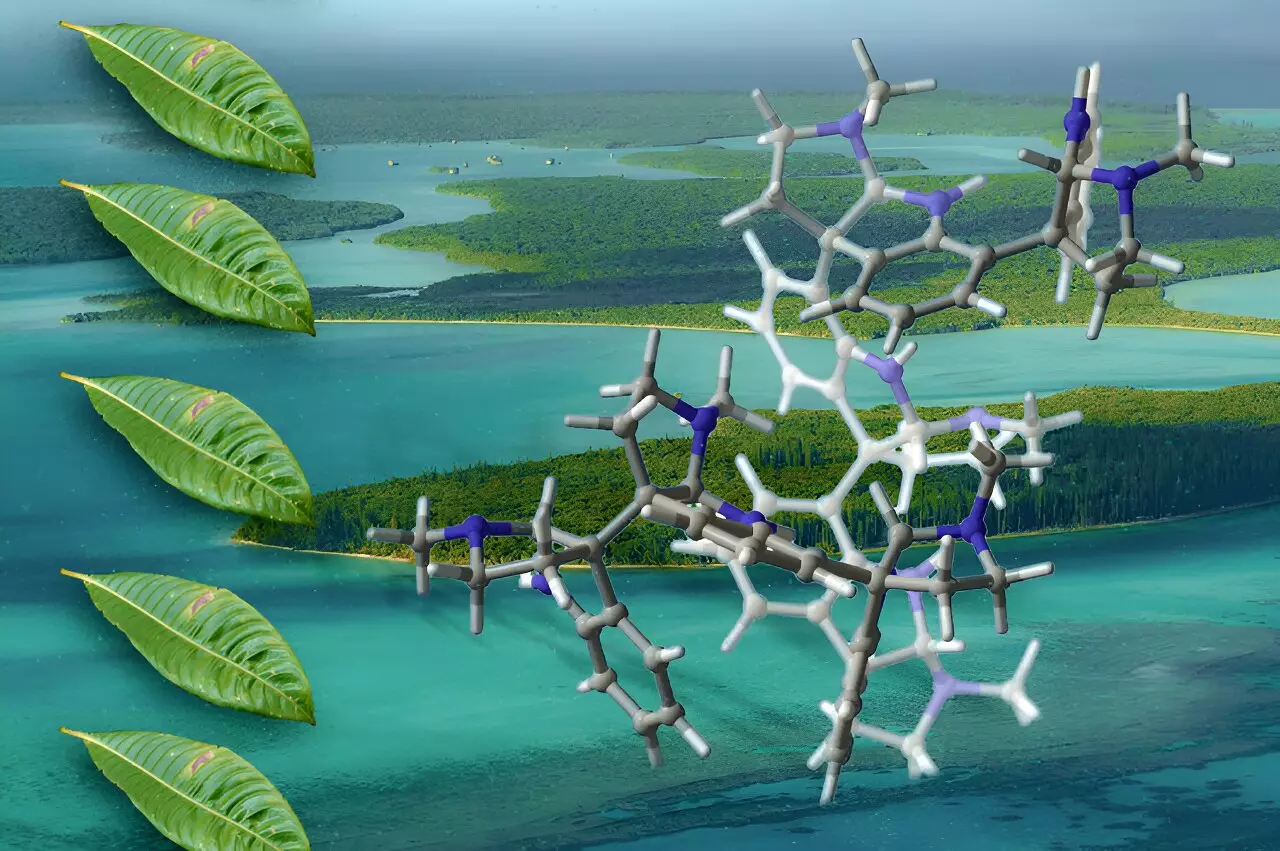
Researchers at the Massachusetts Institute of Technology (MIT) have made a groundbreaking breakthrough in the synthesis of complex organic molecules, specifically oligocyclotryptamines. These compounds, which have shown potential as antibiotics, pain relievers, or even cancer treatments, are primarily derived from plants and are notable for their intricate structure. Unfortunately, their natural abundance is quite limited, presenting a challenge for pharmaceutical research that could unlock new therapeutic avenues. The innovative methodology devised by the MIT team may change the landscape of medicinal chemistry by enabling the reliable production of these complex molecules in the laboratory.
Oligocyclotryptamines are classified as alkaloids, which are organic compounds containing nitrogen and synthesized mostly by plants. These unique molecules encompass multiple tricyclic structures known as cyclotryptamines, interconnected through carbon-carbon bonds. This complexity results in eight different types of oligocyclotryptamines isolated from the Psychotria genus of flowering plants, primarily found in tropical regions. Despite intensive research dating back to the 1950s on smaller derivatives, synthesizing the more intricate oligocyclotryptamines with six or seven fused rings had remained a formidable challenge.
The significance of these compounds lies not only in their rarity but also in their pharmacological potential. With limited natural samples hampering rigorous scientific investigation, the MIT team’s approach holds promise for advancing our understanding of these compounds’ medicinal properties.
Synthesizing oligocyclotryptamines effectively hinges upon overcoming specific structural challenges. At the heart of this complexity lies the formation of bonds between densely clustered carbon atoms within the cyclic structures. Each additional ring introduced into the molecule results in further steric hindrance, rendering many carbon atoms less accessible for usual reactions. To navigate these hurdles, a sophisticated method to control the stereochemistry—arrangement of atoms around the bonding carbon—is essential.
For years, Professor Mohammad Movassaghi and his team have pursued innovative strategies for establishing carbon-carbon bonds in crowded environments. Their 2011 discovery of a method harnessing carbon radicals facilitated a new approach, allowing for the selective joining of carbon atoms, overcoming previous barriers that hindered the synthesis of larger oligocyclotryptamines.
A pivotal method in this groundbreaking discovery is the diazene-directed assembly technique. This process allows researchers to selectively guide chemical reactions through the creation of highly reactive carbon radicals. By first bonding two targeted carbon atoms to nitrogen, the team can excite these fragments with specific wavelengths of light, prompting the release of nitrogen gas. This process exposes two reactive carbon groups that can bond almost instantly.
The implication of this method is profound; Movassaghi’s lab successfully synthesized other significant alkaloids, including communesins found in fungi. This experience laid the groundwork for tackling the larger oligocyclotryptamines, further validating the versatility of the diazene-directed assembly.
With the new synthesis methodology in play, Movassaghi’s team has successfully created molecules featuring six or seven cyclotryptamine rings—an unprecedented feat in organic synthesis. As noted by Professor Seth Herzon from Yale University, this accomplishment represents a significant milestone in the field.
The innovative approach not only allows for the replication of naturally occurring oligocyclotryptamines but also paves the way for the design of novel compounds with tailored characteristics. Researchers can modify the components used in synthesis, leading to new derivatives that may exhibit enhanced medicinal properties compared to their naturally occurring counterparts.
The ramifications of this work extend far beyond mere synthesis. As more oligocyclotryptamines are produced and characterized, pharmaceutical researchers can embark on a deeper explorative journey into their therapeutic potentials. Moving forward, the ability to create reliable supplies of these complex molecules could lead to rigorous studies that decipher their mechanisms of action, as well as identify potential applications in medicine.
In the evolving domain of medicinal chemistry, the synthesis of oligocyclotryptamines marks a remarkable intersection of creativity, innovation, and scientific inquiry. The promise of enhanced therapeutic agents drawn from these plant-derived molecules stands as a testament to the capabilities of modern chemistry and the importance of relentless pursuit in the advancement of human health. By refining the pathways to complex molecular structures, chemists are not only opening doors to unprecedented discoveries but are also laying the foundation for a future rich with potential medicinal breakthroughs.

Leave a Reply