The University of Massachusetts Amherst research team has achieved a significant breakthrough in the modeling and understanding of intrinsically disordered proteins (IDPs) and their spontaneous phase separation. IDPs are critical in numerous biological functions and are linked to cancer, neurodegenerative disorders, and infectious diseases. However, studying and describing the process of phase separation has been challenging. In a recent publication in the Journal of the American Chemical Society, senior author Professor Jianhan Chen presents a novel approach to simulate phase separations mediated by IDPs, shedding light on the molecular level mechanisms involved.
Phase separation has long been recognized as a phenomenon in polymer physics, but it was only about 15 years ago that its prevalence in biology was revealed. Professor Chen explains, “You can look at phase separation with a microscope, but to understand this phenomenon at the molecular level is very difficult.” Recent discoveries indicate that many disordered proteins, including those associated with cancer and neurodegenerative disorders, can drive phase separation. Therefore, understanding the hidden features crucial to the functioning and self-assembly of IDPs is crucial in comprehending the molecular underpinnings of these diseases.
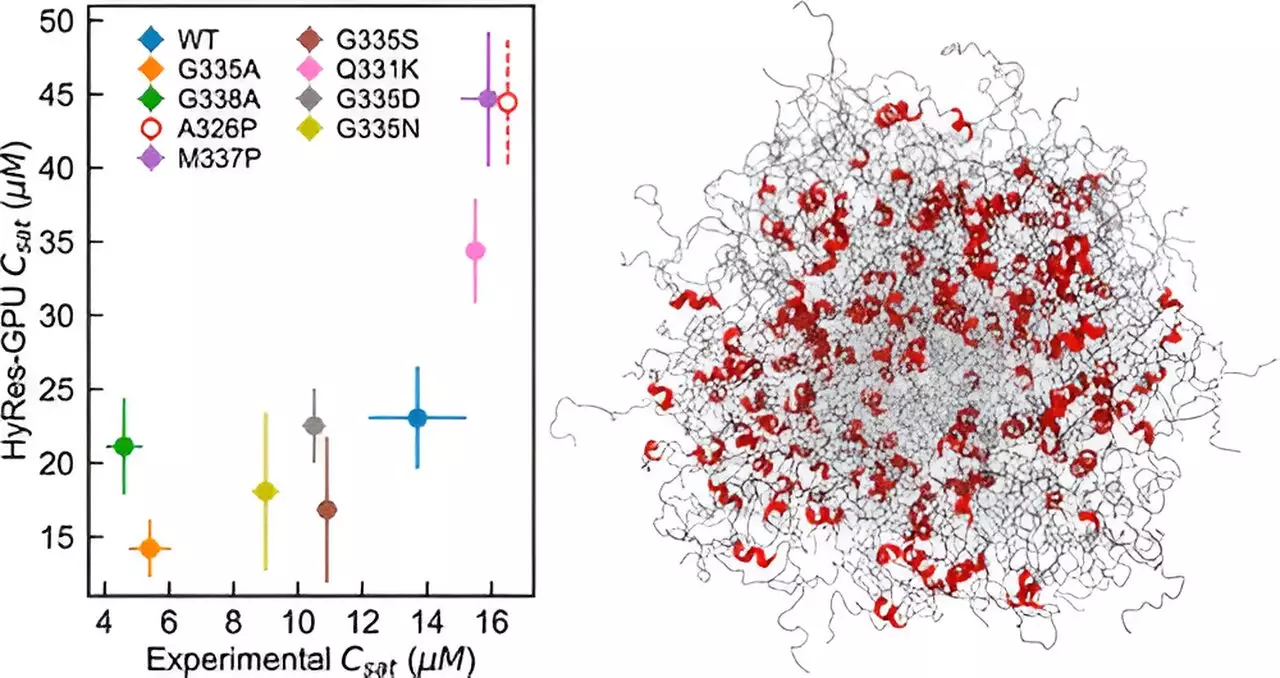
The research team led by Professor Chen has developed a novel computational model, the hybrid resolution (HyRes) force field, which accurately simulates phase separations mediated by IDPs. What sets this model apart is its ability to describe peptide backbone interactions and transient secondary structures while being computationally efficient enough to model liquid-liquid phase separation. This innovation fills a critical gap in the existing capability of computer simulations for IDP phase separation.
Through HyRes simulations, the researchers were able to demonstrate for the first time the factors governing the stability of the condensate of two essential IDPs. Professor Chen remarks, “I actually did not anticipate that it could do such a good job at describing phase separation because it’s a really difficult phenomenon to simulate.” The success of this model opens up opportunities for investigating the impacts of mutations or residual structures on phase separation and advancing therapeutic strategies for diseases related to disordered proteins.
The significance of this work lies in the potential to understand and control the process of phase separation. As Professor Chen highlights, “Important biological processes are believed to occur through phase separation. So, if we can understand better what controls this process, that knowledge will be really powerful if not essential for us to think about controlling phase separation for various scientific and engineering purposes.” Ultimately, this research holds promise for developing therapeutic interventions and engineering applications by gaining a comprehensive understanding of the molecular mechanisms behind phase separation.
Building on their recent breakthrough, Professor Chen and his team will now focus on larger-scale simulations involving more complex biomolecular mixtures. They aim to extend their model to include nucleic acids, as phase separation often involves interactions between disordered proteins and nucleic acids. The exploration of these larger-scale simulations will provide further insights into the intricate mechanisms of phase separation and expand the potential applications of this research.
The University of Massachusetts Amherst research team’s novel approach to simulating phase separations mediated by IDPs represents a significant advance in understanding the molecular mechanisms of biological processes. By developing a unique computational model, the team has successfully shed light on the factors governing condensate stability and paved the way for future research and therapeutic strategies. As the scope of their simulations expands, the team’s findings promise to have a lasting impact on the fields of biomedical research and engineering, ultimately leading to improved treatments for diseases associated with disordered proteins.

Leave a Reply