The scientific community has reached a significant milestone as Stanford researchers have successfully created and stabilized an incredibly rare form of gold, known as Au2+ perovskite. This breakthrough was achieved using a class of crystalline materials called halide perovskites, which hold immense potential for a variety of applications such as solar cells, light sources, and electronic components. What makes this discovery even more remarkable is that the synthesis of the Au2+ perovskite is both swift and straightforward, requiring simple off-the-shelf ingredients at room temperature.
The revelation of a stable Au2+ perovskite took the scientific community by surprise. Hemamala Karunadasa, the senior author of the study and an associate professor of chemistry at the Stanford School of Humanities and Sciences, expressed her initial disbelief: “It was a real surprise that we were able to synthesize a stable material containing Au2+ – I didn’t even believe it at first.” The discovery of this first-of-its-kind material is undoubtedly thrilling due to the intriguing similarities between the gold atoms in the perovskite and the copper atoms found in high-temperature superconductors. Furthermore, heavy atoms with unpaired electrons, like Au2+, exhibit fascinating magnetic effects that lighter atoms do not possess.
Halide perovskites possess highly attractive properties for numerous practical applications, leading researchers to continuously explore and expand this family of materials. Kurt Lindquist, the lead author of the study and a postdoctoral scholar in inorganic chemistry at Princeton University, explained the significance of the unprecedented Au2+ perovskite, stating, “An unprecedented Au2+ perovskite could open some intriguing new avenues.”
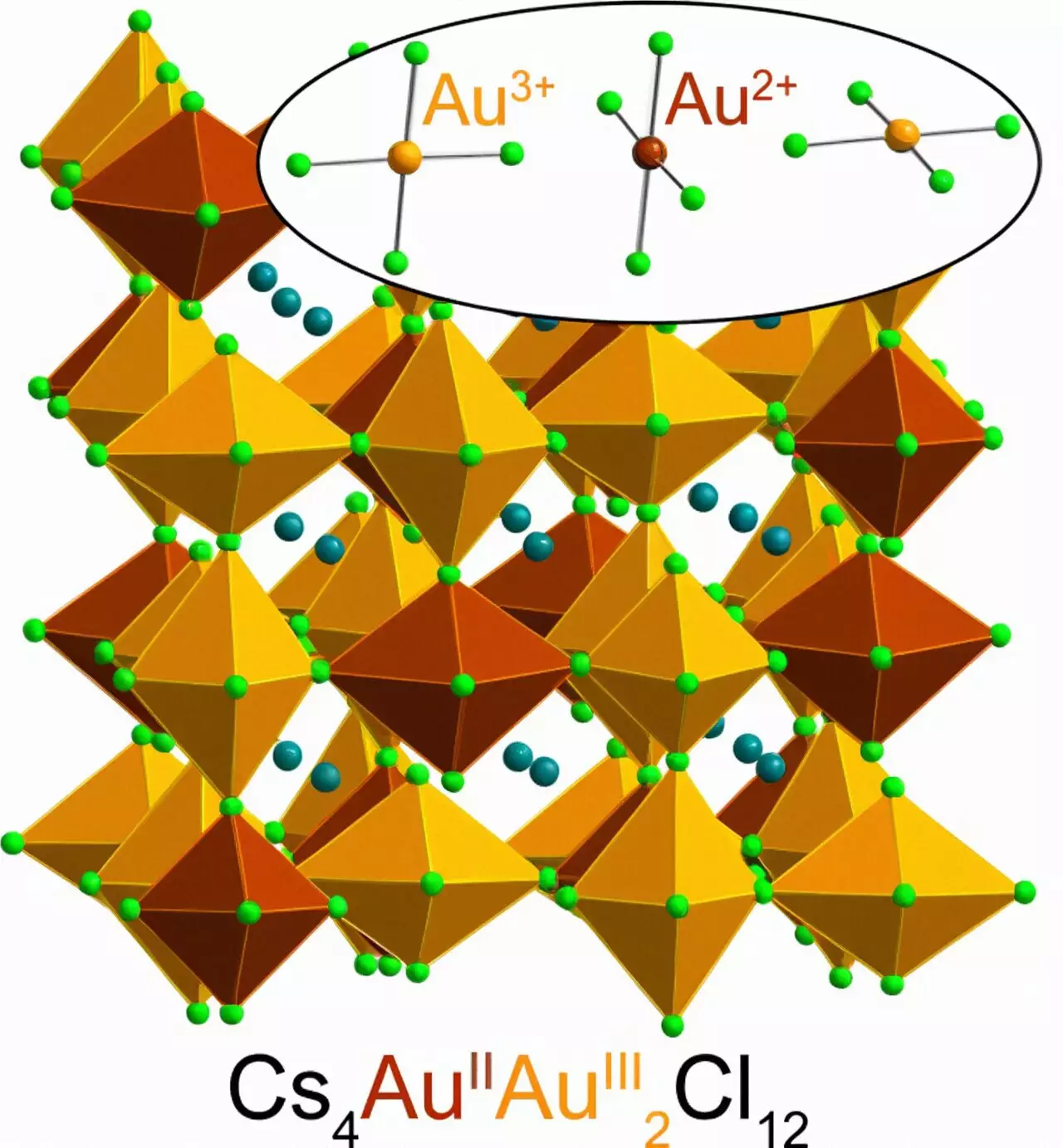
The elemental metal gold has always been highly valued due to its scarcity, malleability, and chemical inertness, making it ideal for jewelry and coins that do not tarnish over time. Moreover, the distinctively rich color of gold further adds to its value. The underlying physics behind gold’s unique appearance also explain why Au2+ is so rare. Albert Einstein’s theory of relativity provides insights into this phenomenon, particularly concerning the relativistic effects that occur when objects, including particles, approach the speed of light. These effects have a profound impact on heavy elements like gold, resulting in the rearrangement of electrons and their energy levels. Consequently, gold absorbs blue light, giving it a yellow appearance. While gold typically occurs as Au1+ and Au3+, losing one or three electrons respectively, the elusive Au2+ is spurned due to the arrangement of gold’s electrons conditioned by relativity.
The Stanford researchers have discovered that Au2+ can be stabilized through a specific molecular configuration. Kurt Lindquist stumbled upon this breakthrough while working on a project focused on magnetic semiconductors for electronic devices. Lindquist mixed cesium chloride and Au3+-chloride in water and added hydrochloric acid to the solution, along with a dash of vitamin C. The reaction that ensued resulted in the donation of an electron by vitamin C to Au3+, effectively forming Au2+. Remarkably, Au2+ remains stable within the solid perovskite structure but not in a solution. The synthesis of this material is remarkably simple and can be accomplished in approximately five minutes at room temperature, yielding a dark green, almost black powder due to the gold it contains.
Recognizing the potential significance of their findings, Lindquist and his team conducted a series of tests, including spectroscopy and X-ray diffraction, to analyze how the perovskite absorbs light and to determine its crystal structure. Other research groups at Stanford, led by Young Lee and Edward Solomon, contributed to studying the behavior of Au2+. The experiments confirmed the presence of Au2+ in the perovskite and added a new chapter to the century-old story of chemistry and physics initially explored by Linus Pauling, a renowned scientist who received the Nobel Prize in Chemistry in 1954. Intriguingly, Pauling also studied the structure of vitamin C, one of the crucial ingredients involved in creating a stable perovskite containing Au2+. This connection to Pauling’s work adds further depth and historical significance to the synthesis of this unique perovskite.
Moving forward, the team of researchers plans to conduct further investigations and make adjustments to the chemistry of the new material. Their hope is that the Au2+ perovskite can be utilized in applications that require magnetism and conductivity, as electrons can hop from Au2+ to Au3+ within the perovskite. This exciting discovery opens up promising possibilities for future scientific advancements and technological breakthroughs. Karunadasa expressed her enthusiasm, stating, “We’re excited to explore what an Au2+ perovskite could do.”
The synthesis and stabilization of Au2+ perovskite represent a significant achievement for the scientific community. The ability to create this elusive material using conventional ingredients at room temperature is extraordinary. With its potential for various applications, including solar cells and electronics components, the Au2+ perovskite opens up a whole new world of possibilities. As researchers continue to study and refine its chemistry, we eagerly anticipate the discoveries and advancements that lie ahead.

Leave a Reply