The production of hydrogen (H2) using solar energy or other renewable energy sources has the potential to be a sustainable and environmentally friendly method. However, most photoelectrochemical water splitting systems developed so far have been inefficient, unstable, or difficult to implement on a large-scale. In a recent study published in Nature Energy, researchers at Ulsan National Institute of Science and Technology (UNIST) proposed a scalable and efficient photoelectrochemical (PEC) system for the production of green hydrogen. This article analyzes the researchers’ innovative approach and its potential implications for the future of hydrogen production.
To develop a viable practical PEC system, a minimum of 10% solar-to-hydrogen (STH) efficiency is required. Previous attempts at photoelectrochemical hydrogen production using stable metal oxides as photoelectrode materials have fallen short of this efficiency target. In search of more efficient alternatives, researchers have explored the potential of photovoltaic (PV) grade materials such as silicon, perovskites, chalcogenides, and III-V material classes. While these materials possess high efficiencies, they can be expensive and unstable when exposed to water. Metal-halide perovskites (MHP), on the other hand, offer a unique combination of high efficiency and low cost. However, their stability in humid conditions and under UV light has been a significant challenge.
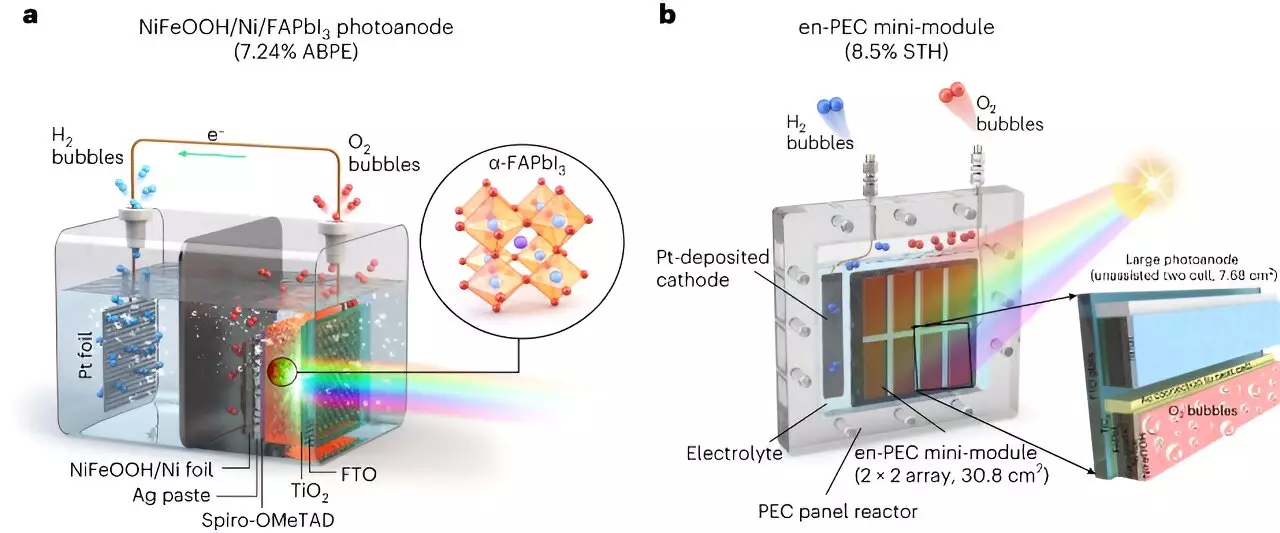
The researchers at UNIST aimed to address the stability challenge and develop photoelectrodes based on MHPs. They used metal-encapsulation and metal-protection techniques, along with the UV-stable formamidinium lead triiodide (FAPbI3) perovskite. By encapsulating the FAPbI3 perovskite with a thick nickel foil deposited with a NiFeOOH catalyst, they achieved stability in water and promoted the oxygen evolution reaction required for water splitting. This approach enabled them to overcome the stability issues associated with MHP materials, making them a potential alternative to traditional photoelectrode materials.
Another crucial challenge for practical applications is scalability. The high efficiency achieved in laboratory-scale cells (

Leave a Reply