The urgent need to combat climate change has prompted researchers to explore innovative methods for carbon dioxide (CO2) conversion into valuable chemicals. A pivotal study led by Georgios Katsoukis and his team at the University of Twente’s Department of Chemical Engineering sheds light on how the surrounding chemical environment of copper electrodes can significantly alter the efficiency of CO2 reduction processes. This research not only emphasizes the role of the reaction environment but also opens up new avenues for the development of more sustainable technologies capable of capturing CO2 emissions.
The Mechanism of CO2 Conversion
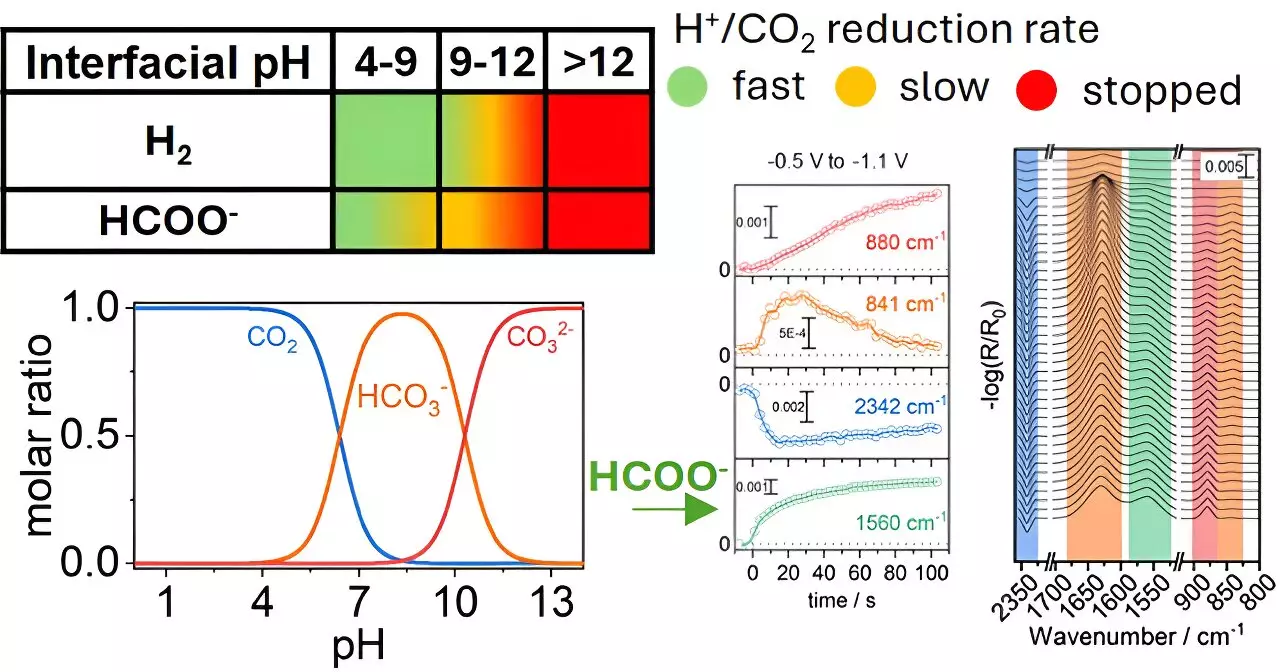
At the heart of the study published in *ACS Catalysis* is the understanding of how CO2 interacts with copper electrodes when submerged in an aqueous solution. Traditional approaches have often focused heavily on the composition and structure of catalyst materials. However, this research indicates that the local chemical conditions—such as pH levels—can dramatically affect the conversion rates of CO2 into formate, a chemical that has broad applications in various industries. The study reveals that by carefully modifying pH levels in the vicinity of the electrodes, researchers can drastically improve the efficiency and selectivity of CO2 reduction reactions.
One of the longstanding challenges in CO2 reduction has been achieving selectivity, given that multiple products can arise from differing reaction conditions. The breakthrough from Katsoukis’s team redefines the discussion around selectivity, underscoring the importance of the chemical environment rather than solely concentrating on catalyst advancements. This fresh perspective urges scientists to recognize that optimizing surrounding conditions is just as vital as enhancing the catalyst’s inherent properties.
Implications for Carbon Capture Technologies
The implications of this research extend far beyond theoretical frameworks. By fine-tuning the chemical environment around copper electrodes, it is possible to not only enhance selectivity toward the desired product, such as formate but also potentially extend the durability of the electrodes themselves. Such advancements hold significant promise in developing more efficient carbon capture systems that contribute to a circular economy—one where CO2 emissions are recycled into useful products instead of merely being expelled into the atmosphere.
The findings from this study provide a critical foundation for future research in CO2 reduction technologies. By advocating for a dual focus on both catalyst optimization and environmental control, the research team lays the groundwork for more effective systems in the battle against climate change. As scientists progress in this field, the insights gained through this study could lead to significant advancements, bringing us one step closer to extensive applications of CO2 reduction that fosters sustainability and resource circularity.
The University of Twente’s groundbreaking work illustrates that the success of CO2 reduction technologies depends not just on the catalysts employed but also on the meticulous optimization of the chemical environment surrounding them. This newfound understanding is a significant stride toward realizing practical solutions for carbon management and contributes substantially to the global pursuit of a sustainable future.

Leave a Reply